| [1] |
FEYNMAN R P. There’s plenty of Room at the Bottom[M]. GILBERT D H. Minaturization. New York: Reinhold Publishing Corporation, 1961: 282–296.
|
| [2] |
ADLEMAN L M. Molecular computation of solutions to combinatorial problems[J]. Science, 1994, 266(5187): 1021–1024. doi: 10.1126/science.7973651
|
| [3] |
BRAICH R S, CHELYAPOV N, JOHNSON C, et al. Solution of a 20-variable 3-SAT problem on a DNA computer[J]. Science, 2002, 296(5567): 499–502. doi: 10.1126/science.1069528
|
| [4] |
BENENSON Y, PAZ-ELIZUR T, ADAR R, et al. Programmable and autonomous computing machine made of biomolecules[J]. Nature, 2001, 414(6862): 430–434. doi: 10.1038/35106533
|
| [5] |
LIU Qinghua, WANG Liman, FRUTOS A G, et al. DNA computing on surfaces[J]. Nature, 2000, 403(6766): 175–179. doi: 10.1038/35003155
|
| [6] |
SAKAMOTO K, GOUZU H, KOMIYA K, et al. Molecular computation by DNA hairpin formation[J]. Science, 2000, 288(5469): 1223–1226. doi: 10.1126/science.288.5469.1223
|
| [7] |
HEAD T, ROZENBERG G, BLADERGROEN R S, et al. Computing with DNA by operating on plasmids[J]. Biosystems, 2000, 57(2): 87–93. doi: 10.1016/S0303-2647(00)00091-5
|
| [8] |
JONOSKA N, KARL S A, and SAITO M. Three dimensional DNA structures in computing[J]. Biosystems, 1999, 52(1/3): 143–153. doi: 10.1016/S0303-2647(99)00041-6
|
| [9] |
ADLEMAN L, CHENG QI, GOEL A, et al. Running time and program size for self-assembled squares[C]. ACM Symposium on Theory of Computing (STOC), New York, USA, 2001. 740–748. doi: 10.1145/380752.380881.
|
| [10] |
MARTIN-VIDE C, PĂUN G, and SALOMAA A. Characterizations of recursively enumerable languages by means of insertion grammars[J]. Theoretical Computer Science, 1998, 205(1/2): 195–205. doi: 10.1016/S0304-3975(97)00079-0
|
| [11] |
LIPTON R J. DNA solution of hard computational problems[J]. Science, 1995, 268(5210): 542–545. doi: 10.1126/science.7725098
|
| [12] |
ROTHEMUND P. A DNA and restriction enzyme implementation of Turing machines[C]. Proceedings of the DIMACS Workshop, Princeton, USA, 1995.
|
| [13] |
OGIHARA M and RAY A. Simulating Boolean circuits on a DNA computer[J]. Algorithmica, 1999, 25(2/3): 239–250. doi: 10.1007/PL00008276
|
| [14] |
BENENSON Y, GIL B, BEN-DOR U et al. An autonomous molecular computer for logical control of gene expression[J]. Nature, 2004, 429(6990): 423–429. doi: 10.1038/nature02551
|
| [15] |
XU Jin. Probe machine[J]. IEEE Transactions on Neural Networks and Learning Systems, 2016, 27(7): 1405–1416. doi: 10.1109/TNNLS.2016.2555845
|
| [16] |
WINFREE E, LIU Furong, WENZLER L A, et al. Design and self-assembly of two-dimensional DNA crystals[J]. Nature, 1998, 394(6693): 539–544. doi: 10.1038/28998
|
| [17] |
YAN Hao, PARK S H, FINKELSTEIN G et al. DNA-templated self-assembly of protein arrays and highly conductive nanowires[J]. Science, 2003, 301(5641): 1882–1884. doi: 10.1126/science.1089389
|
| [18] |
ROTHEMUND P W K. Folding DNA to create nanoscale shapes and patterns[J]. Nature, 2006, 440(7082): 297–302. doi: 10.1038/nature04586
|
| [19] |
HAN Dongran, PAL S, NANGREAVE J, et al. DNA origami with complex curvatures in Three-Dimensional space[J]. Science, 2011, 332(6027): 342–346. doi: 10.1126/science.1202998
|
| [20] |
ISHIKAWA D, SUZUKI Y, KUROKAWA C, et al. DNA origami Nanoplate-based emulsion with Nanopore function[J]. Angewandte Chemie-International Edition, 2019, 58(43): 15299–15303. doi: 10.1002/anie.201908392
|
| [21] |
JOURNOT C M A, RAMAKRISHNA V, WALLACE M I, et al. Modifying membrane morphology and interactions with DNA origami clathrin-mimic networks[J]. ACS Nano, 2019, 13(9): 9973–9979. doi: 10.1021/acsnano.8b07734
|
| [22] |
LI Na, SHANG Yingxu, XU Rui et al. Precise organization of metal and metal Oxide Nanoclusters into arbitrary patterns on DNA origami[J]. Journal of the American Chemical Society, 2019, 141(45): 17968–17972. doi: 10.1021/jacs.9b09308
|
| [23] |
JOHNSON J A, DEHANKAR A, WINTER J O, et al. Reciprocal control of hierarchical DNA origami-Nanoparticle assemblies[J]. NANO Letters, 2019, 19(12): 8469–8475. doi: 10.1021/acs.nanolett.9b02786
|
| [24] |
KLEIN W P, THOMSEN R P, TURNER K B, et al. Enhanced catalysis from multienzyme cascades assembled on a DNA origami triangle[J]. ACS Nano, 2019, 13(12): 13677–13689. doi: 10.1021/acsnano.9b05746
|
| [25] |
BERENGUT J F, BERENGUT J C, DOYE J P K, et al. Design and synthesis of pleated DNA origami nanotubes with adjustable diameters[J]. Nucleic Acids Research, 2019, 47(22): 11963–11975. doi: 10.1093/nar/gkz1056
|
| [26] |
HAHN J, CHOU L Y T, SØRENSEN R S, et al. Extrusion of RNA from a DNA-origami-based Nanofactory[J]. ACS Nano, 2020, 14(2): 1550–1559. doi: 10.1021/acsnano.9b06466
|
| [27] |
ANASTASSACOS F M, ZHAO Zhao, ZENG Yang, et al. Glutaraldehyde cross-linking of Oligolysines coating DNA origami greatly reduces susceptibility to nuclease degradation[J]. Journal of the American Chemical Society, 2020, 142(7): 3311–3315. doi: 10.1021/jacs.9b11698
|
| [28] |
BARTNIK K, BARTH A, PILO-PAIS M, et al. A DNA origami platform for single-pair Förster resonance energy transfer investigation of DNA-DNA interactions and ligation[J]. Journal of the American Chemical Society, 2020, 142(2): 815–825. doi: 10.1021/jacs.9b09093
|
| [29] |
殷志祥, 唐震, 张强, 等. 基于DNA折纸基底的与非门计算模型[J]. 电子与信息学报, 2020, 42(6): 1355–1364. doi: 10.11999/JEIT190825YIN Zhixiang, TANG Zhen, ZHANG Qiang, et al. NAND gate computational model based on the DNA origami template[J]. Journal of Electronics &Information Technology, 2020, 42(6): 1355–1364. doi: 10.11999/JEIT190825
|
| [30] |
王君珂, 印珏, 牛人杰, 等. DNA计算与DNA纳米技术[J]. 电子与信息学报, 2020, 42(6): 1313–1325. doi: 10.11999/JEIT190826WANG Junke, YIN Jue, NIU Renjie, et al. DNA computing and DNA Nanotechnology[J]. Journal of Electronics &Information Technology, 2020, 42(6): 1313–1325. doi: 10.11999/JEIT190826
|
| [31] |
XU Jin, QIANG Xiaoli, ZHANG Kai, et al. A DNA computing model for the Graph Vertex Coloring problem based on a probe graph[J]. Engineering, 2018, 4(1): 61–77. doi: 10.1016/j.eng.2018.02.011
|





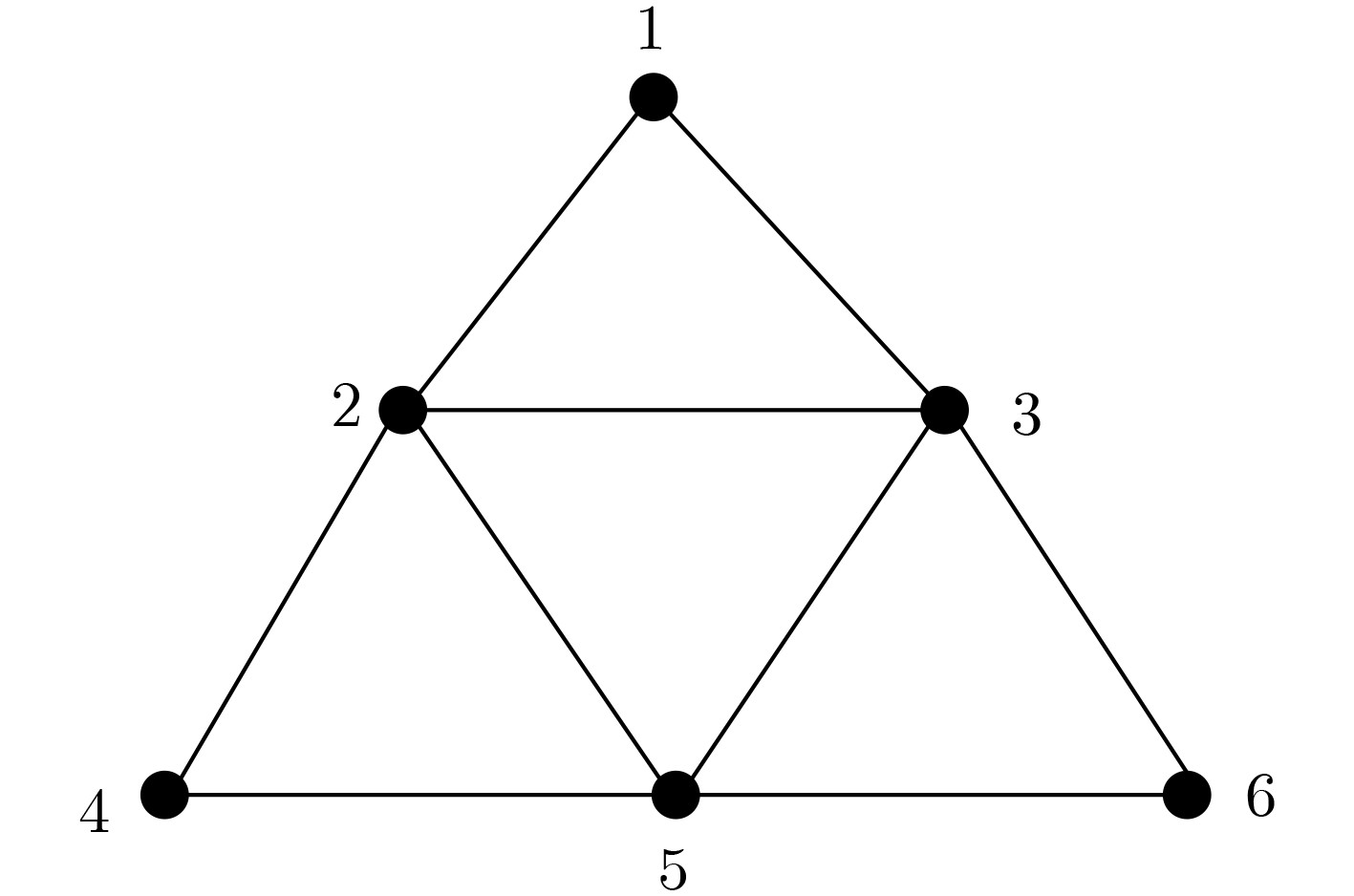
 下载:
下载:
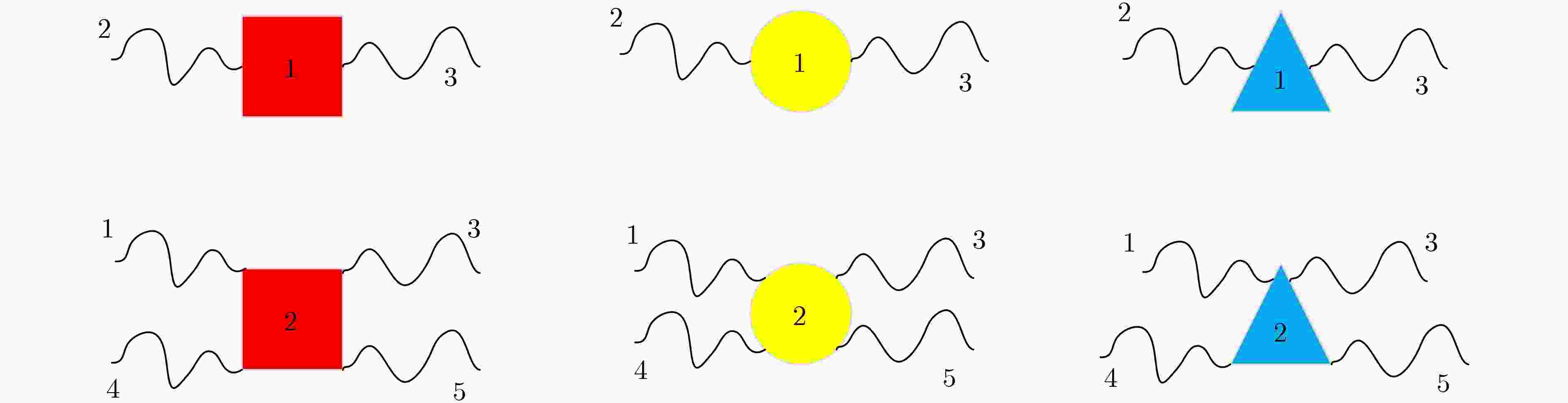
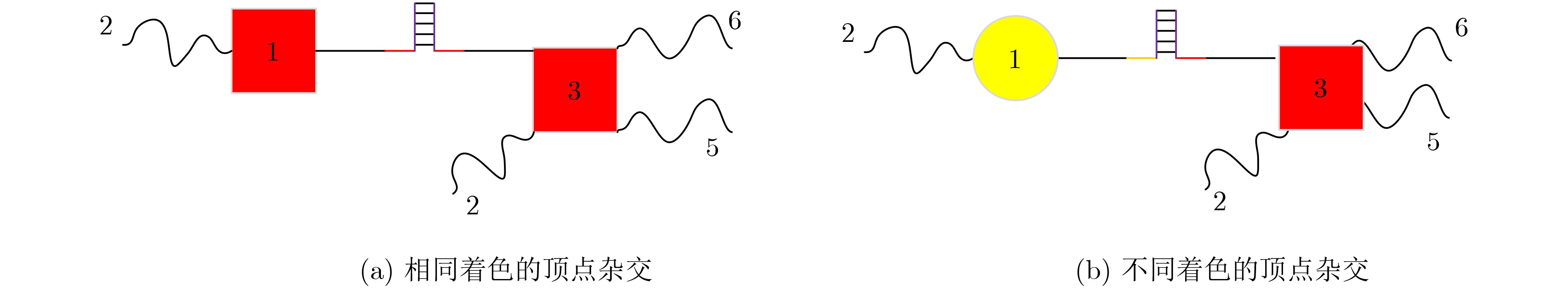
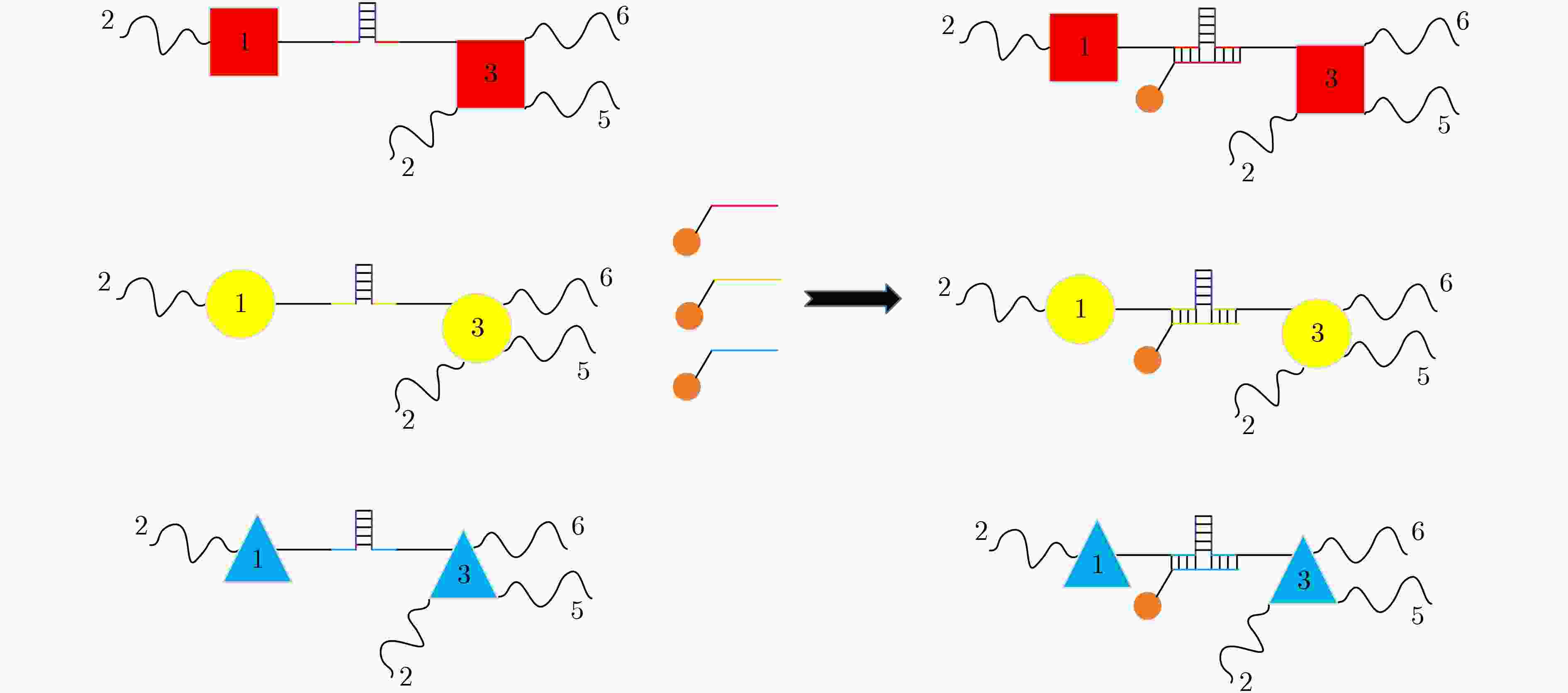


 下载:
下载:
