Detection and Interaction Analysis of Place Cell Firing Information in Dual Brain Regions of Awake Active Rats
-
摘要: 连续探测自由活动大鼠的神经活动对于研究大脑功能具有重要意义,但同时也是一个挑战。该文研究旨在通过双脑区探测提供全面的大脑活动信息。为此,设计了一种符合双脑区形状的四探针微电极阵列(MEA),并使用聚吡咯/银纳米线(PPy/AgNW)纳米复合材料进行表面修饰。优化后,PPy/AgNW纳米复合材料修饰的MEA展现出低阻抗(53.01 ± 2.59 kΩ),增强了信号采集性能。进一步研究了PPy/AgNW纳米复合材料修饰的MEA的稳定性。经过
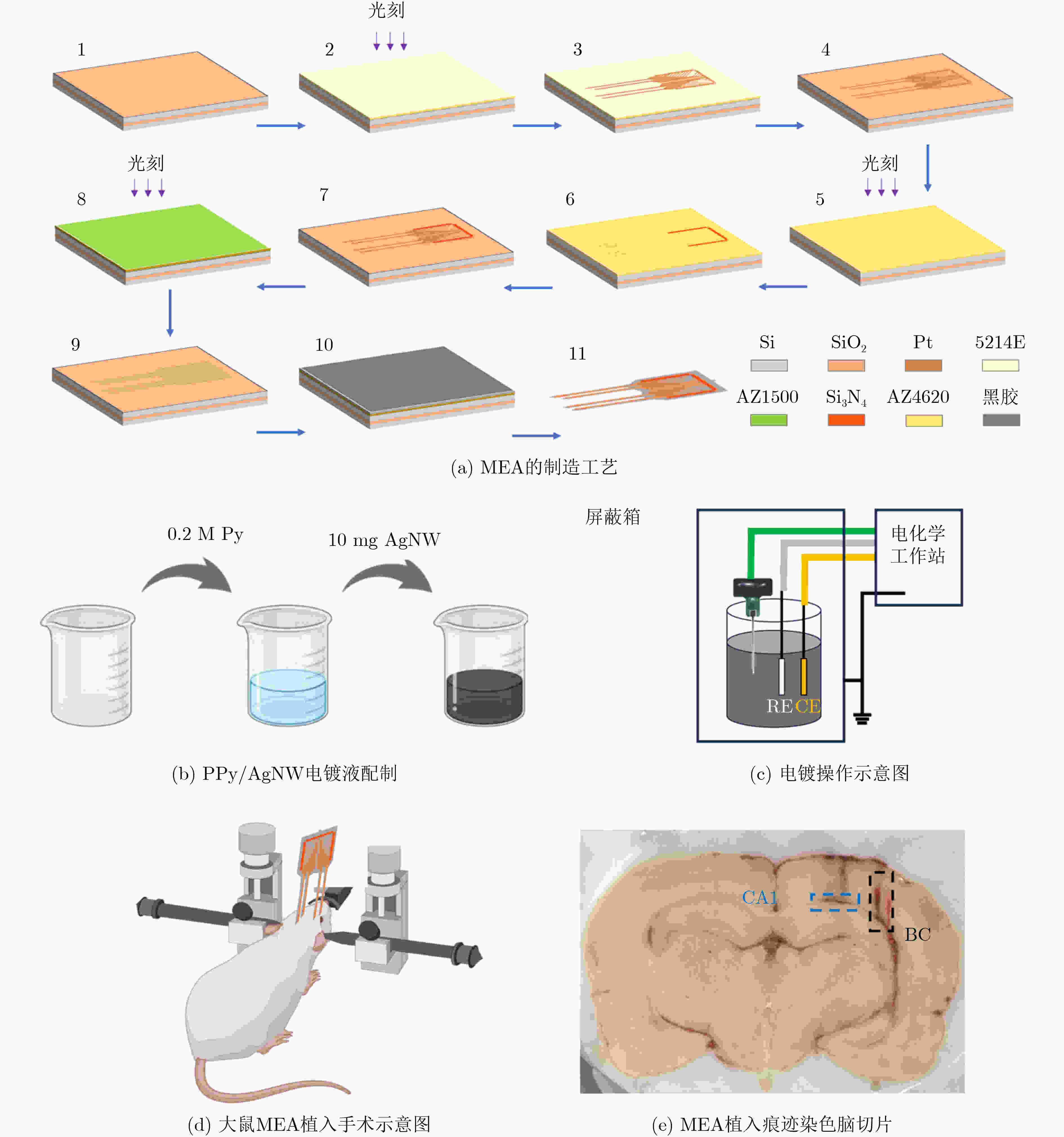
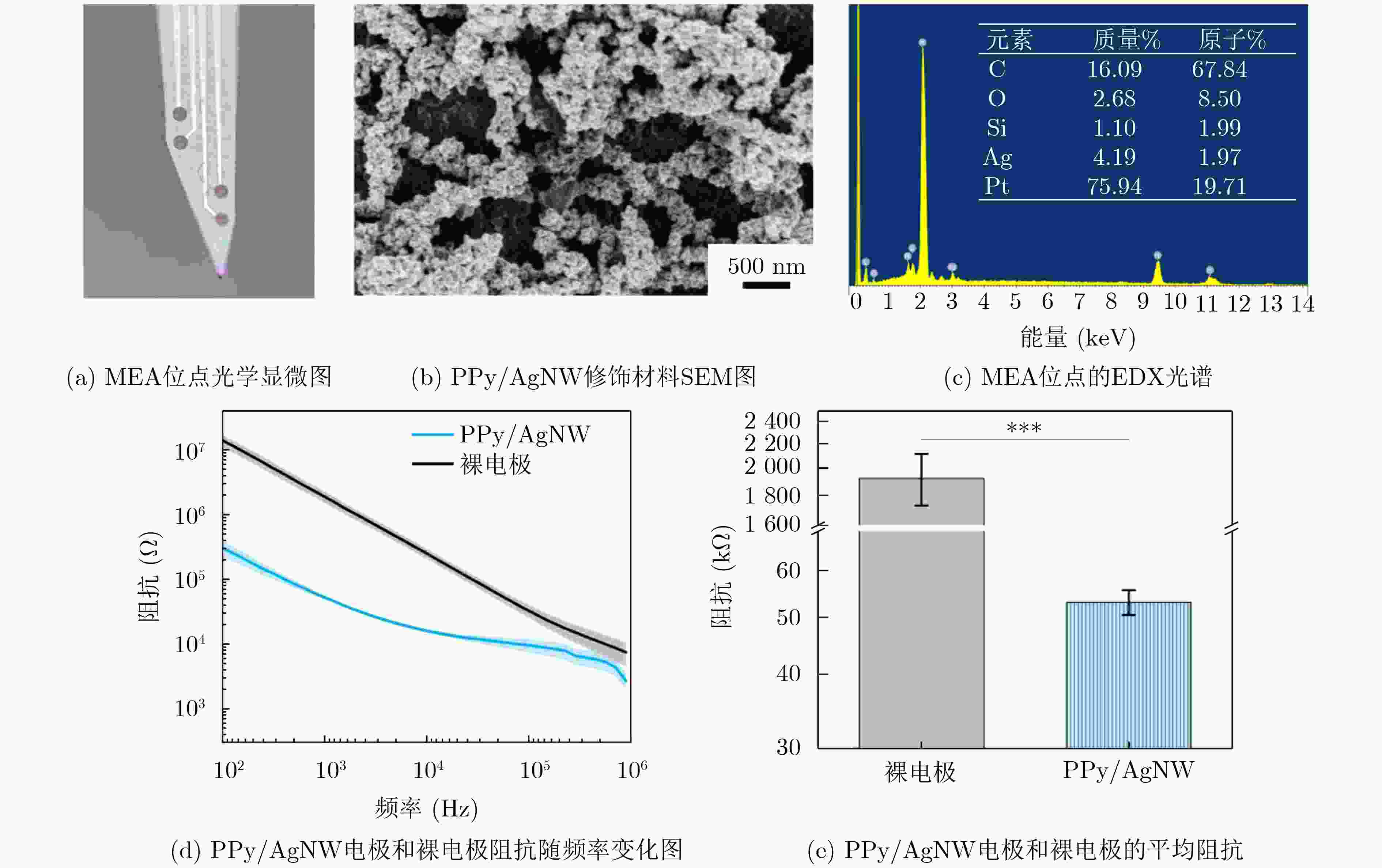
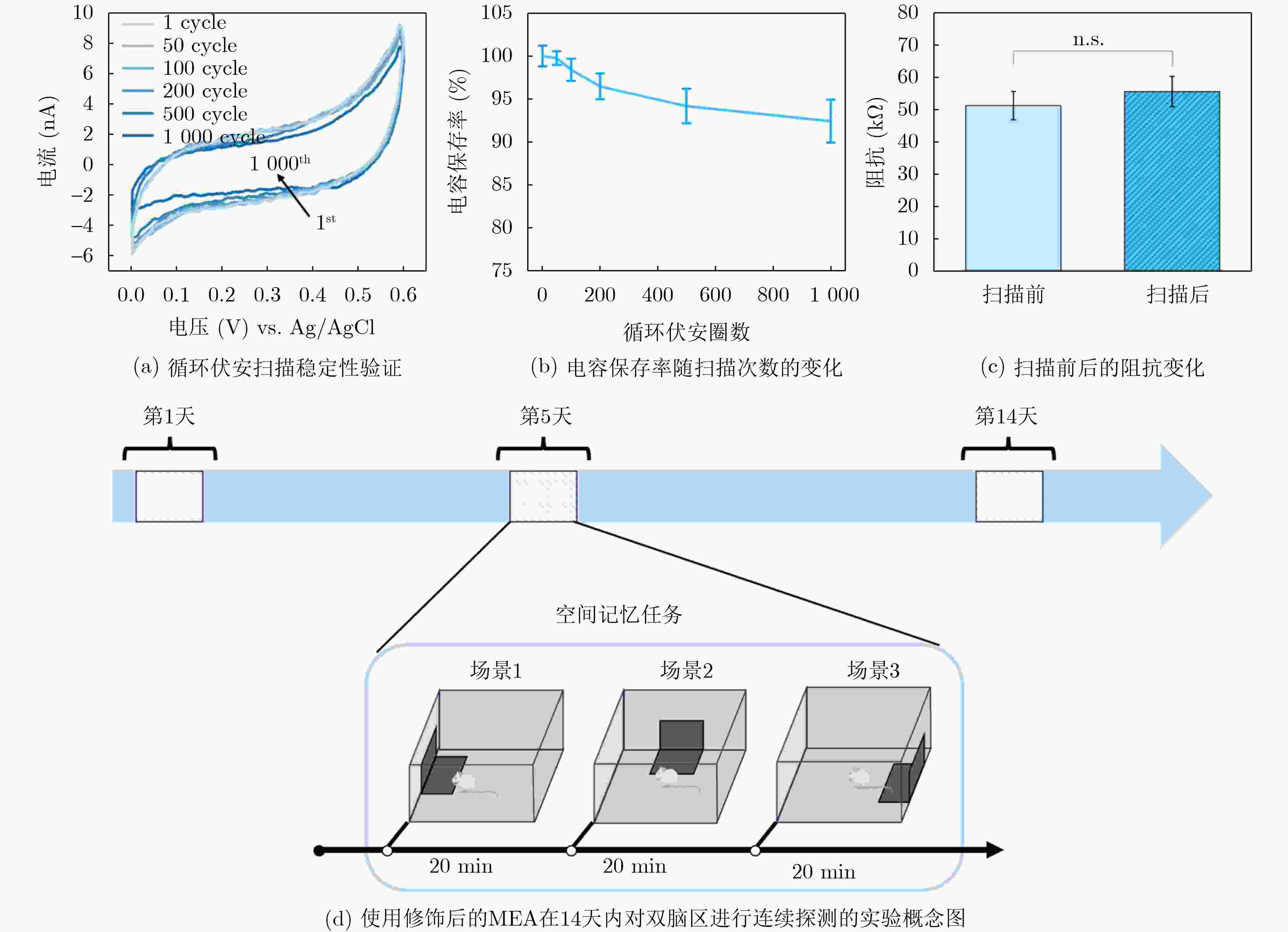
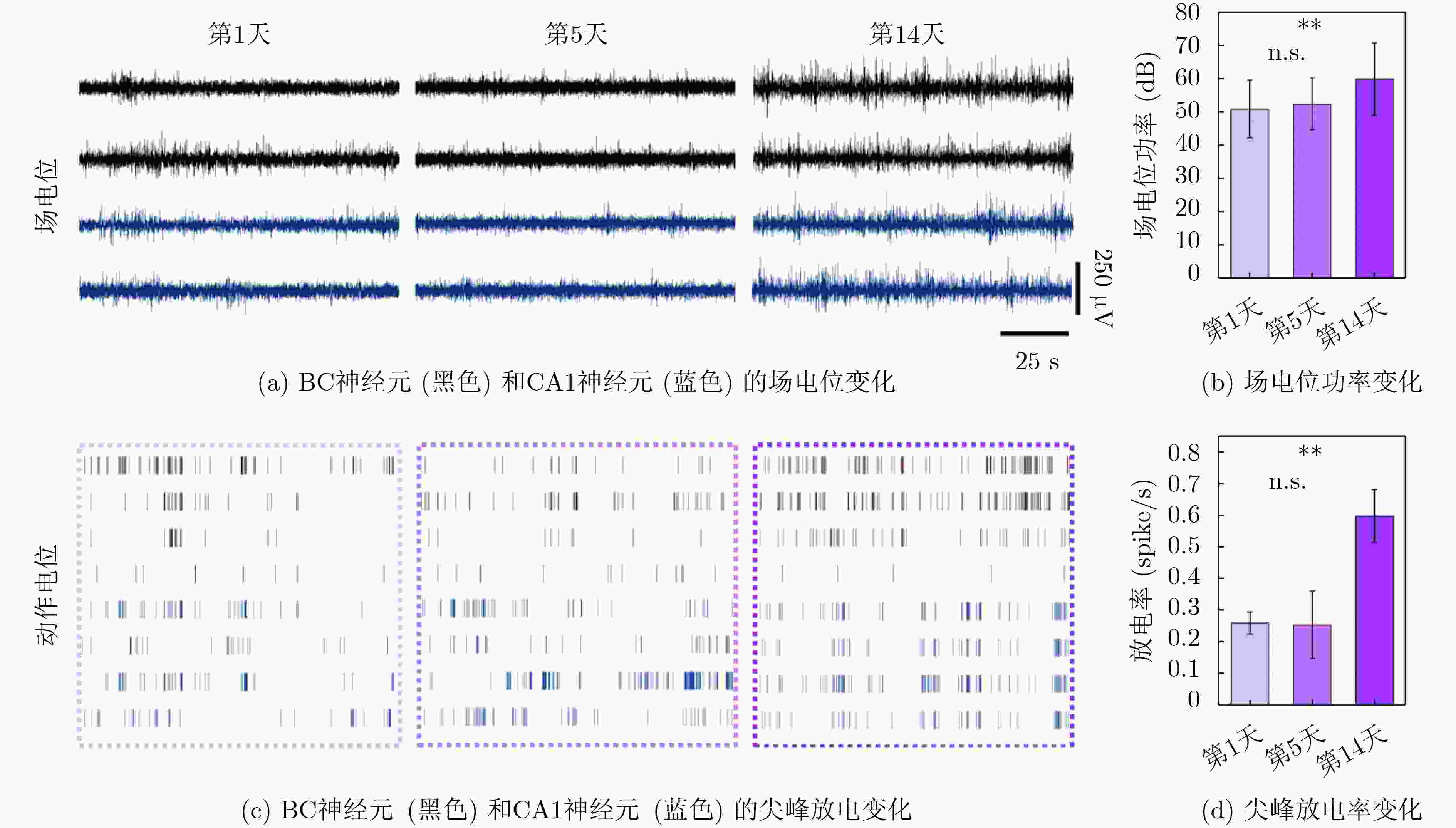
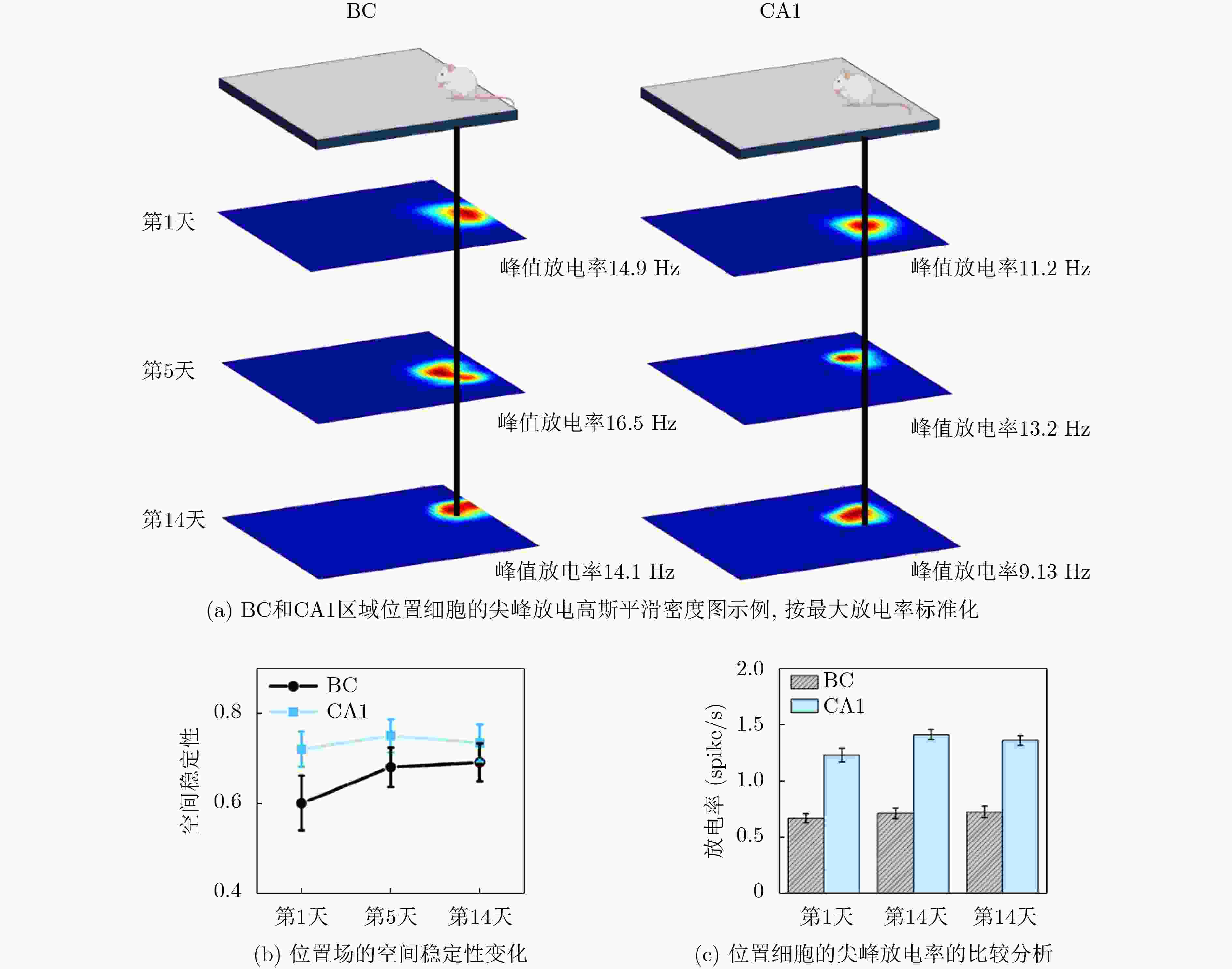
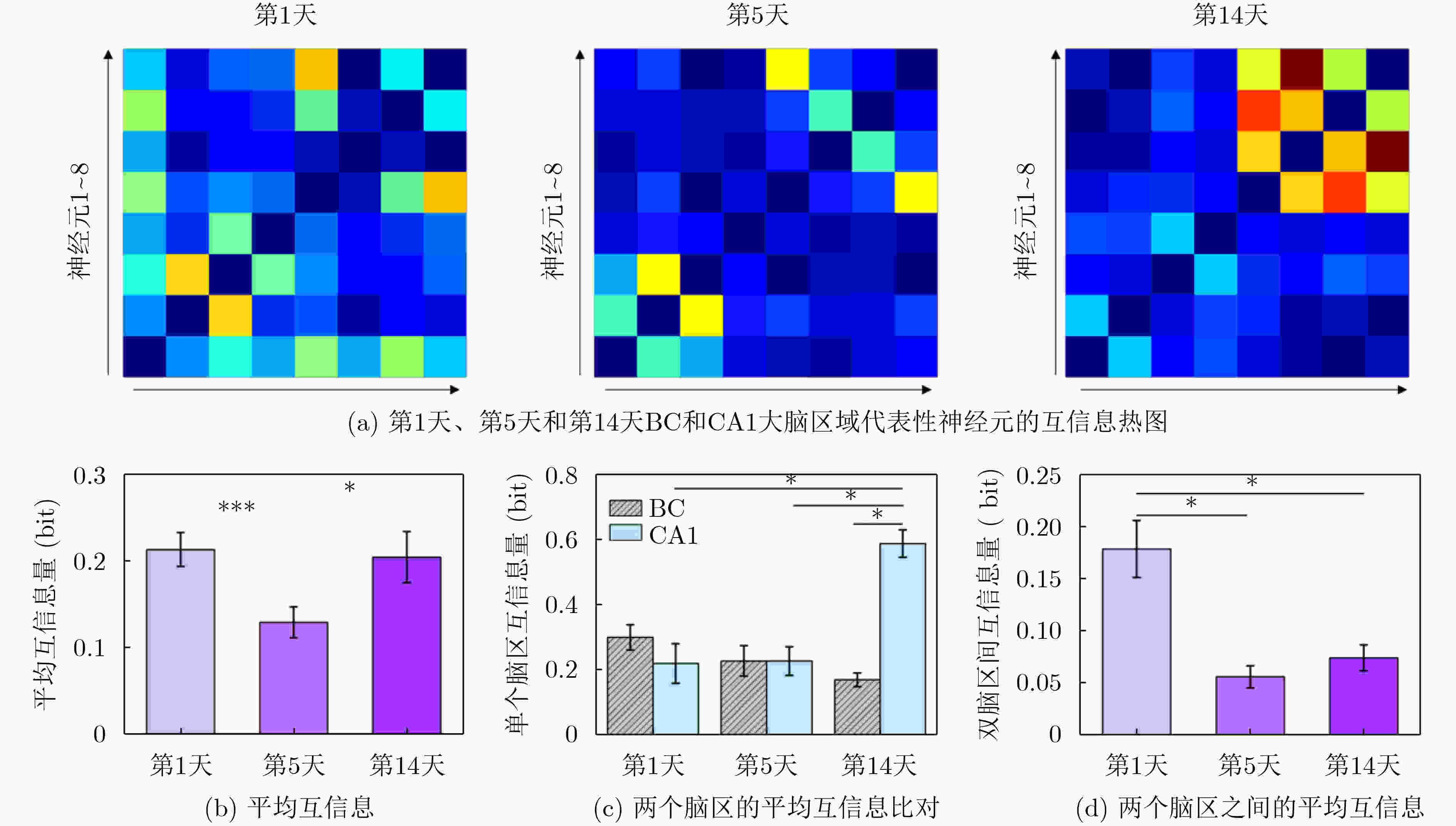
1000 次循环伏安扫描后,电容保持率为92.51% ± 2.21%,阻抗未显著增加,表明其具有长期体内探测的高稳定性。MEA植入大鼠相应脑区后不影响其自由活动,并成功检测到连续两周的空间认知过程中尖峰信号和局部场电位信号,确认了记录神经元中存在位置细胞。为了评估神经元动态的变化,我们计算了神经元之间的互信息、特别关注单脑区内以及双脑区之间的交互信息。在初始记忆阶段,观察到双脑区间显著的信息交换,可能与记忆存储有关。总之,本研究成功地使用纳米材料修饰的MEA实现了对移动大鼠双脑区的动态探测,揭示了参与空间记忆过程的神经动态。这一见解对于更深入地理解大脑活动机制和相关疾病具有重要意义。Abstract:Objective Continuous monitoring of neural activities in free-moving rats is essential for understanding brain function but presents significant challenges regarding the stability and biocompatibility of the detection device. This study aims to provide comprehensive data on brain activity by simultaneously monitoring two brain regions. This approach is crucial for elucidating the neural encoding differences within these regions and the information exchange between them, both of which are integral to spatial memory and navigation processes. Spatial navigation is a fundamental behavior in rats, vital for their survival and interaction with their environment. Central to this behavior are place cells—neurons that selectively respond to an animal’s location, forming the basis of spatial memory and navigation. This study focuses on the hippocampal CA1 region and the Barrel Cortex (BC), both of which are critical for spatial processing. By monitoring these regions simultaneously, the aim is to uncover the neural dynamics underlying spatial memory formation and retrieval. Understanding these dynamics provides insights into the neural mechanisms of spatial cognition and memory, which are fundamental to higher cognitive functions and are often disrupted in neurological disorders such as Alzheimer’s disease and schizophrenia. Methods To achieve dual brain region monitoring, a four-electrode MicroElectrode Array (MEA) is designed to conform to the shape of the dual brain regions and is surface-modified with a Polypyrrole/Silver Nanowire (PPy/AgNW) nanocomposite material. Each probe of the MEA consists of eight recording sites with a diameter of 20 μm and one reference site. The MEA is fabricated using Microelectromechanical Systems (MEMS) technology and modified via an electrochemical deposition process. The PPy/AgNW nanocomposite modification is selected for its low impedance and high biocompatibility, which are critical for stable, long-term recordings. The deposition of PPy/AgNW is carried out using cyclic voltammetry. The stability of the modified MEA is assessed by cyclic voltammetry in phosphate-buffered saline to simulate in vivo charge/discharge processes. The MEA is then implanted into the CA1 and BC regions of rats, and neural activities are recorded during a two-week spatial memory task. Spike signals are analyzed to identify place cells and assess their firing patterns, while Local Field Potential (LFP) power is measured to evaluate overall neural activity. Mutual information analysis is performed to quantify the interaction between the two brain regions. The experimental setup includes a behavior arena where rats perform spatial navigation tasks, with continuous neural signal recording using the modified MEA. Results and Discussions The PPy/AgNW-modified MEA exhibits low impedance (53.01 ± 2.59 kΩ) at 1 kHz ( Fig. 2 ). This low impedance is critical for high-fidelity signal acquisition, enabling the detection of subtle neural activities. The stability of the MEA is evaluated through1000 cycles of cyclic voltammetry scanning, demonstrating high capacitance retention (92.51 ± 2.21%) and no significant increase in impedance (Fig. 3 ). These results suggest that the MEA maintains stable performance over extended periods, which is essential for long-term in vivo monitoring. The modified MEA successfully detects neural activities from the BC and CA1 regions over the two-week period. The average firing rates and LFP power in both regions progressively increase, indicating enhanced neural activity as the rats become more familiar with the spatial memory task (Fig. 4 ). This increase suggests that the rats’ spatial memory and navigation abilities improve over time, likely due to increased familiarity with the environment and task requirements. Place cells are identified in the recorded neurons, confirming the presence of spatially selective neuronal activity (Fig. 5 ). The identification of place cells is a key finding, as these neurons are fundamental to spatial memory and navigation. Additionally, the spatial stability of place cells in the CA1 region is higher than in the BC region, indicating functional differences between these areas in spatial memory processing (Fig. 5 ). This suggests that the CA1 region plays a more critical role in spatial memory consolidation. Mutual information analysis reveals significant information exchange between the dual brain regions during the initial memory phase, suggesting a role in memory storage (Fig. 6 ). This inter-regional communication is crucial for understanding how spatial information is processed and stored in the brain. The observed increase in mutual information over time indicates that the interaction between the BC and CA1 regions becomes more pronounced as the rats engage in spatial navigation, highlighting the dynamic nature of neural interactions during memory formation and retrieval.Conclusions This study successfully demonstrated continuous dual brain region monitoring in freely moving rats using a PPy/AgNW-modified MEA. The findings reveal dynamic interactions between the BC and CA1 regions during spatial memory tasks and highlight the importance of place cells in memory formation. Monitoring neural activities in dual brain regions over extended periods provides new insights into the neural basis of spatial memory and navigation. The results suggest that the CA1 region plays a critical role in spatial memory consolidation, while the BC region also contributes to spatial processing. This distinction highlights the value of studying multiple brain regions simultaneously to gain a comprehensive understanding of neural processes. The PPy/AgNW-modified MEA serves as a powerful tool for investigating the complex neural mechanisms underlying spatial cognition and memory, with potential applications in related neurological disorders. -
Key words:
- Brain-computer interface /
- Nanomaterials /
- MicroElectrode Array (MEA) /
- Place cell
-
[1] PEREIRA A, RIBEIRO S, WIEST M, et al. Processing of tactile information by the hippocampus[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(46): 18286–18291. doi: 10.1073/pnas.0708611104. [2] LEWIS C M, BOEHLER C, LILJEMALM R, et al. Recording quality is systematically related to electrode impedance[J]. Advanced Healthcare Materials, 2024, 13(24): 2303401. doi: 10.1002/adhm.202303401. [3] YANG Gucheng, WANG Yiding, XU Zhaojie, et al. PtNPs/PEDOT: PSS-modified microelectrode arrays for detection of the discharge of head direction cells in the retrosplenial cortex of rats under dissociation between visual and vestibular inputs[J]. Biosensors, 2023, 13(5): 496. doi: 10.3390/bios13050496. [4] LU Botao, FAN Penghui, LI Ming, et al. Detection of neuronal defensive discharge information transmission and characteristics in periaqueductal gray double-subregions using PtNP/PEDOT: PSS modified microelectrode arrays[J]. Microsystems & Nanoengineering, 2023, 9: 70. doi: 10.1038/s41378-023-00546-8. [5] XU Zhaojie, MO Fan, YANG Gucheng, et al. Grid cell remapping under three-dimensional object and social landmarks detected by implantable microelectrode arrays for the medial entorhinal cortex[J]. Microsystems & Nanoengineering, 2022, 8: 104. doi: 10.1038/s41378-022-00436-5. [6] POO C, AGARWAL G, BONACCHI N, et al. Spatial maps in piriform cortex during olfactory navigation[J]. Nature, 2022, 601(7894): 595–599. doi: 10.1038/s41586-021-04242-3. [7] FLOSSMANN T and ROCHEFORT N L. Spatial navigation signals in rodent visual cortex[J]. Current Opinion in Neurobiology, 2021, 67: 163–173. doi: 10.1016/j.conb.2020.11.004. [8] LI Jing, CUI Mengjie, WANG Li, et al. Nonionic waterborne polyurethane/polypyrrole/silver nanowires coating film with high-efficient electromagnetic interference shielding[J]. Chemical Physics Letters, 2022, 804: 139882. doi: 10.1016/j.cplett.2022.139882. [9] YANG Yan, DENG Yu, XU Shihong, et al. PPy/SWCNTs-modified microelectrode array for learning and memory model construction through electrical stimulation and detection of in vitro hippocampal neuronal network[J]. ACS Applied Bio Materials, 2023, 6(9): 3414–3422. doi: 10.1021/acsabm.3c00105. [10] GENER T, PEREZ-MENDEZ L, and SANCHEZ-VIVES M V. Tactile modulation of hippocampal place fields[J]. Hippocampus, 2013, 23(12): 1453–1462. doi: 10.1002/hipo.22198. [11] MOSER E I, KROPFF E, and MOSER M B. Place cells, grid cells, and the brain’s spatial representation system[J]. Annual Review of Neuroscience, 2008, 31: 69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [12] XU Longqian, HU Chenxuan, HUANG Qi, et al. Trends and recent development of the microelectrode arrays (MEAs)[J]. Biosensors and Bioelectronics, 2021, 175: 112854. doi: 10.1016/j.bios.2020.112854. [13] LONG Xiaoyang and ZHANG Shengjia. A novel somatosensory spatial navigation system outside the hippocampal formation[J]. Cell Research, 2021, 31(6): 649–663. doi: 10.1038/s41422-020-00448-8. [14] ROBERTS T P, KERN F B, FERNANDO C, et al. Encoding temporal regularities and information copying in hippocampal circuits[J]. Scientific Reports, 2019, 9(1): 19036. doi: 10.1038/s41598-019-55395-1. [15] JAMALI M, GRANNAN B, CAI Jing, et al. Semantic encoding during language comprehension at single-cell resolution[J]. Nature, 2024, 631(8021): 610–616. doi: 10.1038/s41586-024-07643-2. -





 下载:
下载:


 下载:
下载:
