| [1] |
ZHIRNOV V, ZADEGAN R M, SANDHU G S, et al. Nucleic acid memory[J]. Nature Materials, 2016, 15(4): 366–370. doi: 10.1038/nmat4594.
|
| [2] |
CEZE L, NIVALA J, and STRAUSS K. Molecular digital data storage using DNA[J]. Nature Reviews Genetics, 2019, 20(8): 456–466. doi: 10.1038/s41576-019-0125-3.
|
| [3] |
BENENSON Y, PAZ-ELIZUR T, ADAR R, et al. Programmable and autonomous computing machine made of biomolecules[J]. Nature, 2001, 414(6862): 430–434. doi: 10.1038/35106533.
|
| [4] |
王君珂, 印珏, 牛人杰, 等. DNA计算与DNA纳米技术[J]. 电子与信息学报, 2020, 42(6): 1313–1325. doi: 10.11999/JEIT190826.WANG Junke, YIN Jue, NIU Renjie, et al. DNA computing and DNA nanotechnology[J]. Journal of Electronics & Information Technology, 2020, 42(6): 1313–1325. doi: 10.11999/JEIT190826.
|
| [5] |
PENCHOVSKY R and BREAKER R R. Computational design and experimental validation of oligonucleotide-sensing allosteric ribozymes[J]. Nature Biotechnology, 2005, 23(11): 1424–1433. doi: 10.1038/nbt1155.
|
| [6] |
QIAN Lulu and WINFREE E. Scaling up digital circuit computation with DNA strand displacement cascades[J]. Science, 2011, 332(6034): 1196–1201. doi: 10.1126/science.1200520.
|
| [7] |
殷志祥, 唐震, 张强, 等. 基于DNA折纸基底的与非门计算模型[J]. 电子与信息学报, 2020, 42(6): 1355–1364. doi: 10.11999/JEIT190825.YIN Zhixiang, TANG Zhen, ZHANG Qiang, et al. NAND gate computational model based on the DNA origami template[J]. Journal of Electronics & Information Technology, 2020, 42(6): 1355–1364. doi: 10.11999/JEIT190825.
|
| [8] |
CLELLAND C T, RISCA V, and BANCROFT C. Hiding messages in DNA microdots[J]. Nature, 1999, 399(6736): 533–534. doi: 10.1038/21092.
|
| [9] |
JONOSKA N, PĂUN G, and ROZENBERG G. Aspects of Molecular Computing: Essays Dedicated to Tom Head on the Occasion of His 70th Birthday[M]. Berlin: Springer, 2004: 167–188. doi: 10.1007/b94864.
|
| [10] |
LEIER A, RICHTER C, BANZHAF W, et al. Cryptography with DNA binary strands[J]. Biosystems, 2000, 57(1): 13–22. doi: 10.1016/S0303-2647(00)00083-6.
|
| [11] |
LUSTGARTEN O, MOTIEI L, and MARGULIES D. User authorization at the molecular scale[J]. ChemPhysChem, 2017, 18(13): 1678–1687. doi: 10.1002/cphc.201700506.
|
| [12] |
NUMMELIN S, KOMMERI J, KOSTIAINEN M A, et al. Evolution of structural DNA nanotechnology[J]. Advanced Materials, 2018, 30(24): 1703721. doi: 10.1002/adma.201703721.
|
| [13] |
SEEMAN N C. Nucleic acid junctions and lattices[J]. Journal of Theoretical Biology, 1982, 99(2): 237–247. doi: 10.1016/0022-5193(82)90002-9.
|
| [14] |
FU T J and SEEMAN N C. DNA double-crossover molecules[J]. Biochemistry, 1993, 32(13): 3211–3220. doi: 10.1021/bi00064a003.
|
| [15] |
LABEAN T H, YAN Hao, KOPATSCH J, et al. Construction, analysis, ligation, and self-assembly of DNA triple crossover complexes[J]. Journal of the American Chemical Society, 2000, 122(9): 1848–1860. doi: 10.1021/ja993393e.
|
| [16] |
REISHUS D, SHAW B, BRUN Y, et al. Self-assembly of DNA double-double crossover complexes into high-density, doubly connected, planar structures[J]. Journal of the American Chemical Society, 2005, 127(50): 17590–17591. doi: 10.1021/ja0557177.
|
| [17] |
KE Yonggang, LIU Yan, ZHANG Junping, et al. A study of DNA tube formation mechanisms using 4-, 8-, and 12-Helix DNA nanostructures[J]. Journal of the American Chemical Society, 2006, 128(13): 4414–4421. doi: 10.1021/ja058145z.
|
| [18] |
ROTHEMUND P W K. Folding DNA to create nanoscale shapes and patterns[J]. Nature, 2006, 440(7082): 297–302. doi: 10.1038/nature04586.
|
| [19] |
OBER M F, BAPTIST A, WASSERMANN L, et al. In situ small-angle X-ray scattering reveals strong condensation of DNA origami during silicification[J]. Nature Communications, 2022, 13(1): 5668. doi: 10.1038/s41467-022-33083-5.
|
| [20] |
DAI Xinpei, CHEN Xiaoliang, JING Xinxin, et al. DNA origami-encoded integration of heterostructures[J]. Angewandte Chemie International Edition, 2022, 61(11): e202114190. doi: 10.1002/anie.202114190.
|
| [21] |
ZHAO Yumeng, ZHANG Chao, YANG Linlin, et al. Programmable and site-specific patterning on DNA origami templates with heterogeneous condensation of silver and silica[J]. Small, 2021, 17(47): 2103877. doi: 10.1002/smll.202103877.
|
| [22] |
HANNEWALD N, WINTERWERBER P, ZECHEL S, et al. DNA origami meets polymers: A powerful tool for the design of defined nanostructures[J]. Angewandte Chemie International Edition, 2021, 60(12): 6218–6229. doi: 10.1002/anie.202005907.
|
| [23] |
ARYAL B R, RANASINGHE D R, PANG Chao, et al. Annealing of polymer-encased nanorods on DNA origami forming metal–semiconductor nanowires: Implications for nanoelectronics[J]. ACS Applied Nano Materials, 2021, 4(9): 9094–9103. doi: 10.1021/acsanm.1c01682.
|
| [24] |
MADSEN M, BAKKE M R, GUDNASON D A, et al. A single molecule polyphenylene-vinylene photonic wire[J]. ACS Nano, 2021, 15(6): 9404–9411. doi: 10.1021/acsnano.0c10922.
|
| [25] |
YANG Yunqi, LU Qinyi, HUANG Chaomin, et al. Programmable site-specific functionalization of DNA origami with polynucleotide brushes[J]. Angewandte Chemie International Edition, 2021, 60(43): 23241–23247. doi: 10.1002/anie.202107829.
|
| [26] |
KAHN J S, XIONG Yan, HUANG J, et al. Cascaded enzyme reactions over a three-dimensional, wireframe DNA origami scaffold[J]. JACS Au, 2022, 2(2): 357–366. doi: 10.1021/jacsau.1c00387.
|
| [27] |
WANG S T, MINEVICH B, LIU Jianfang, et al. Designed and biologically active protein lattices[J]. Nature Communications, 2021, 12(1): 3702. doi: 10.1038/s41467-021-23966-4.
|
| [28] |
ZHAO Shuai, TIAN Run, WU Jun, et al. A DNA origami-based aptamer nanoarray for potent and reversible anticoagulation in hemodialysis[J]. Nature Communications, 2021, 12(1): 358. doi: 10.1038/s41467-020-20638-7.
|
| [29] |
孙彤, 刘文静, 张萍, 等. 基于DNA折纸的单个链霉亲和素分子的原子力显微术高分辨成像[J]. 核技术, 2019, 42(4): 040501. doi: 10.11889/j.0253-3219.2019.hjs.42.040501.SUN Tong, LIU Wenjing, ZHANG Ping, et al. High-resolution imaging of single-molecule streptavidin using atomic force microscopy based on DNA origami[J]. Nuclear Techniques, 2019, 42(4): 040501. doi: 10.11889/j.0253-3219.2019.hjs.42.040501.
|
| [30] |
YE Jingjing, AFTENIEVA O, BAYRAK T, et al. Complex metal nanostructures with programmable shapes from simple DNA building blocks[J]. Advanced Materials, 2021, 33(29): 2100381. doi: 10.1002/adma.202100381.
|
| [31] |
RYSSY J, NATARAJAN A K, WANG Jinhua, et al. Light-responsive dynamic DNA-origami-based plasmonic assemblies[J]. Angewandte Chemie, 2021, 133(11): 5923–5927. doi: 10.1002/ange.202014963.
|
| [32] |
MA Yuxuan, LU Zhangwei, JIA Bin, et al. DNA origami as a nanomedicine for targeted rheumatoid arthritis therapy through reactive oxygen species and nitric oxide scavenging[J]. ACS Nano, 2022, 16(8): 12520–12531. doi: 10.1021/acsnano.2c03991.
|
| [33] |
COMBERLATO A, KOGA M M, NÜSSING S, et al. Spatially controlled activation of toll-like receptor 9 with DNA-based nanomaterials[J]. Nano Letters, 2022, 22(6): 2506–2513. doi: 10.1021/acs.nanolett.2c00275.
|
| [34] |
KNAPPE G A, WAMHOFF E C, READ B J, et al. In situ covalent functionalization of DNA origami virus-like particles[J]. ACS Nano, 2021, 15(9): 14316–14322. doi: 10.1021/acsnano.1c03158.
|
| [35] |
CHATTERJEE G, DALCHAU N, MUSCAT R A, et al. A spatially localized architecture for fast and modular DNA computing[J]. Nature Nanotechnology, 2017, 12(9): 920–927. doi: 10.1038/nnano.2017.127.
|
| [36] |
THUBAGERE A J, LI Wei, JOHNSON R F, et al. A cargo-sorting DNA robot[J]. Science, 2017, 357(6356): eaan6558. doi: 10.1126/science.aan6558.
|
| [37] |
LIU Fengsong, LI Na, SHANG Yingxu, et al. A DNA-based plasmonic nanodevice for cascade signal amplification[J]. Angewandte Chemie International Edition, 2022, 61(22): e202114706. doi: 10.1002/anie.202114706.
|
| [38] |
ZHANG Yinan, WANG Fei, CHAO Jie, et al. DNA origami cryptography for secure communication[J]. Nature Communications, 2019, 10(1): 5469. doi: 10.1038/s41467-019-13517-3.
|
| [39] |
STALLINGS W. The advanced encryption standard[J]. Cryptologia, 2002, 26(3): 165–188. doi: 10.1080/0161-110291890876.
|





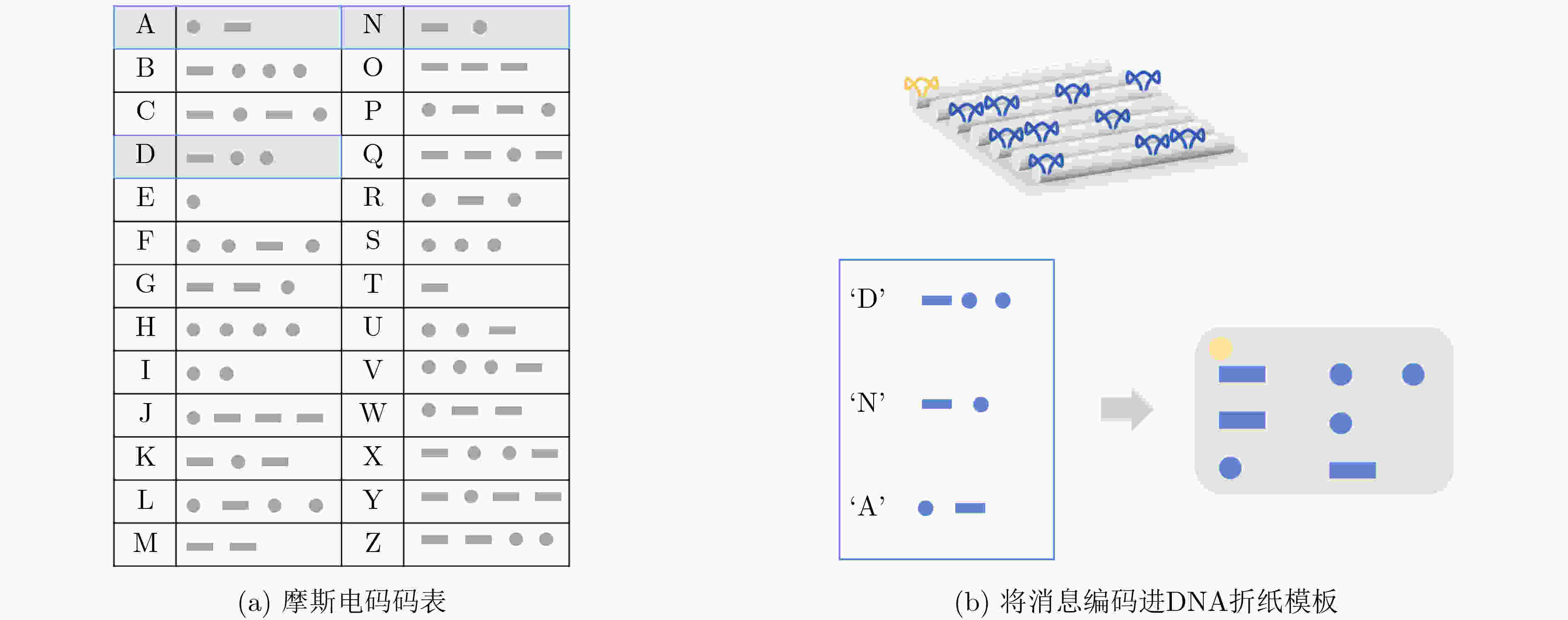
 下载:
下载:
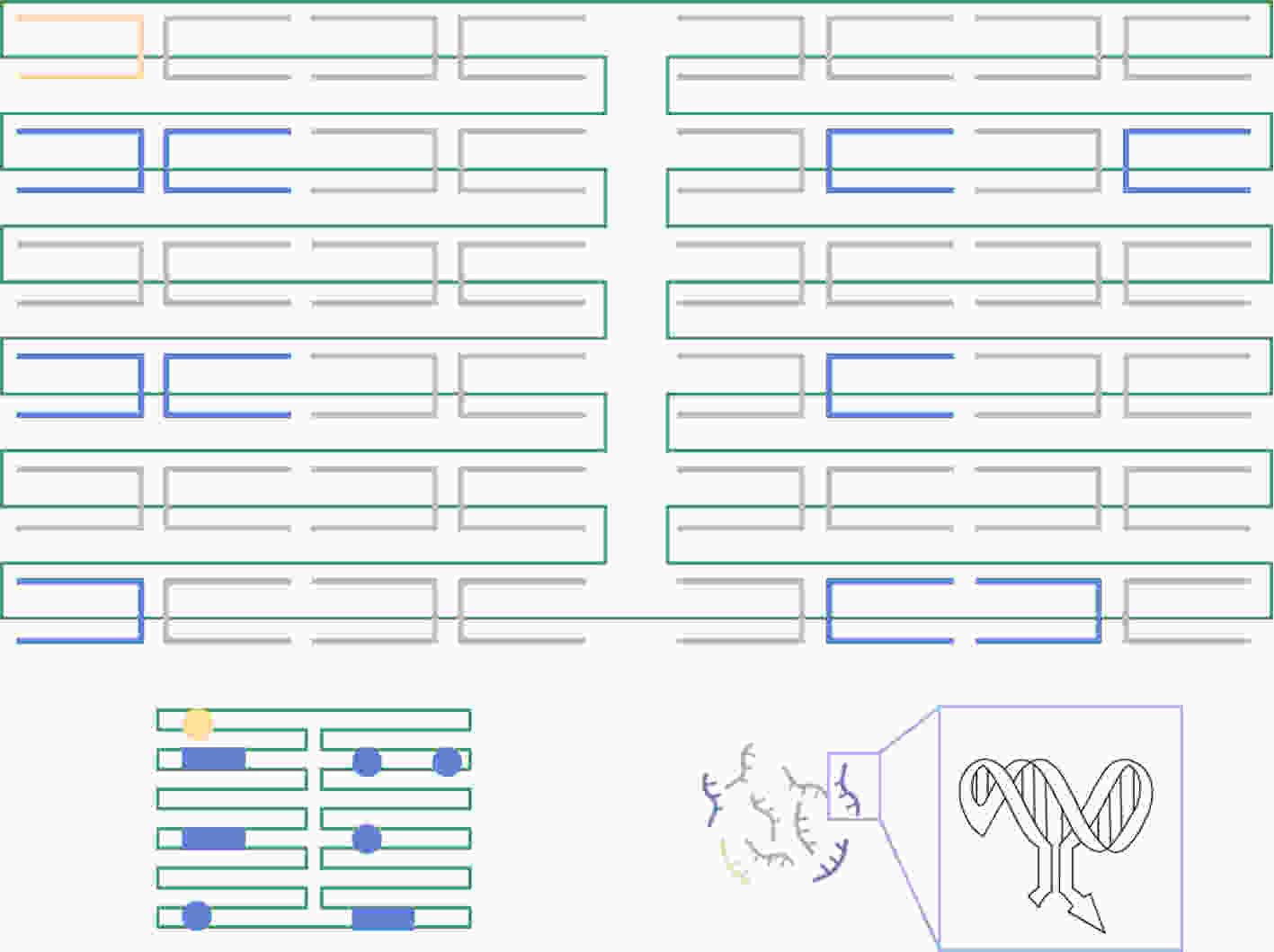
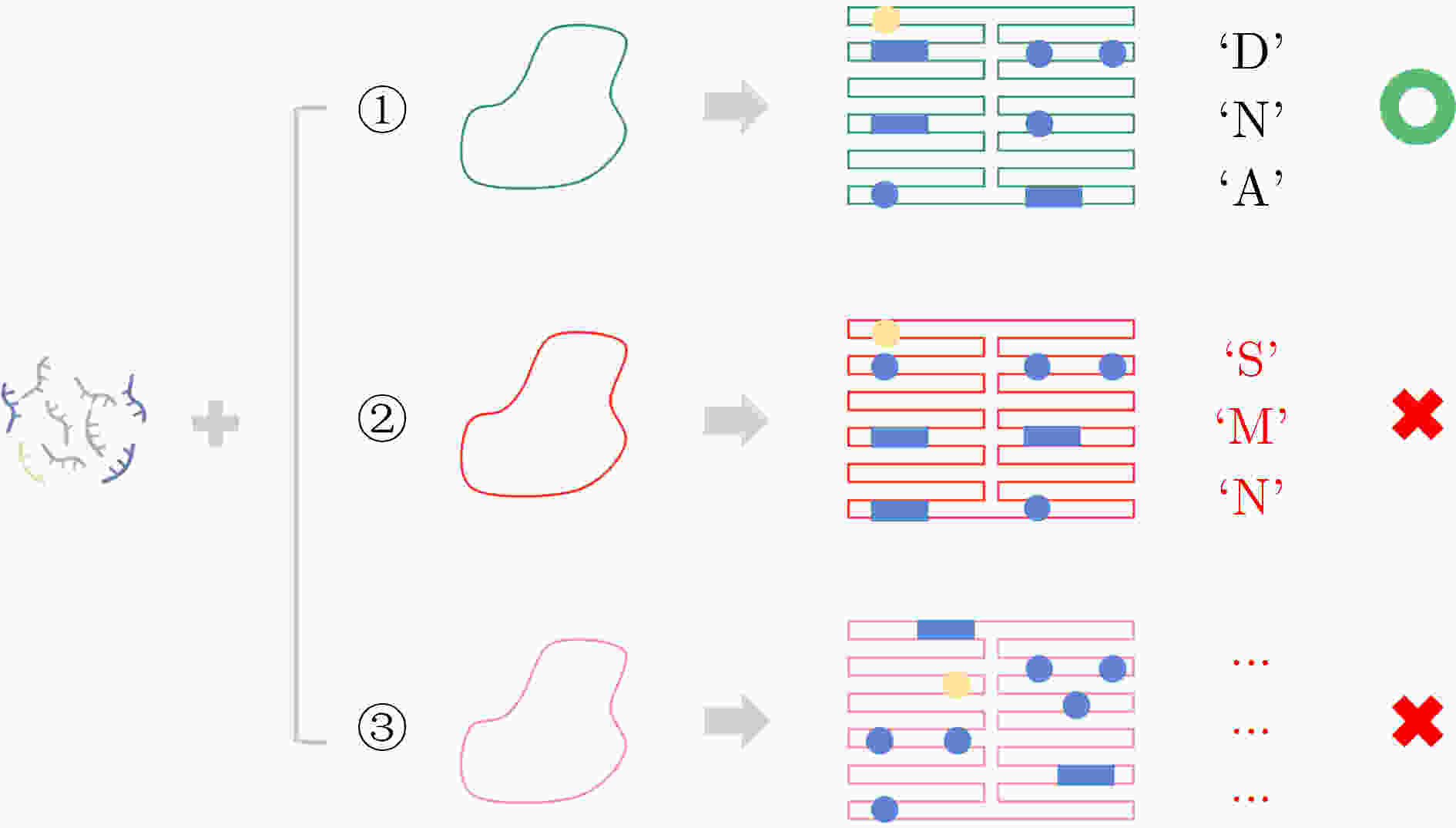

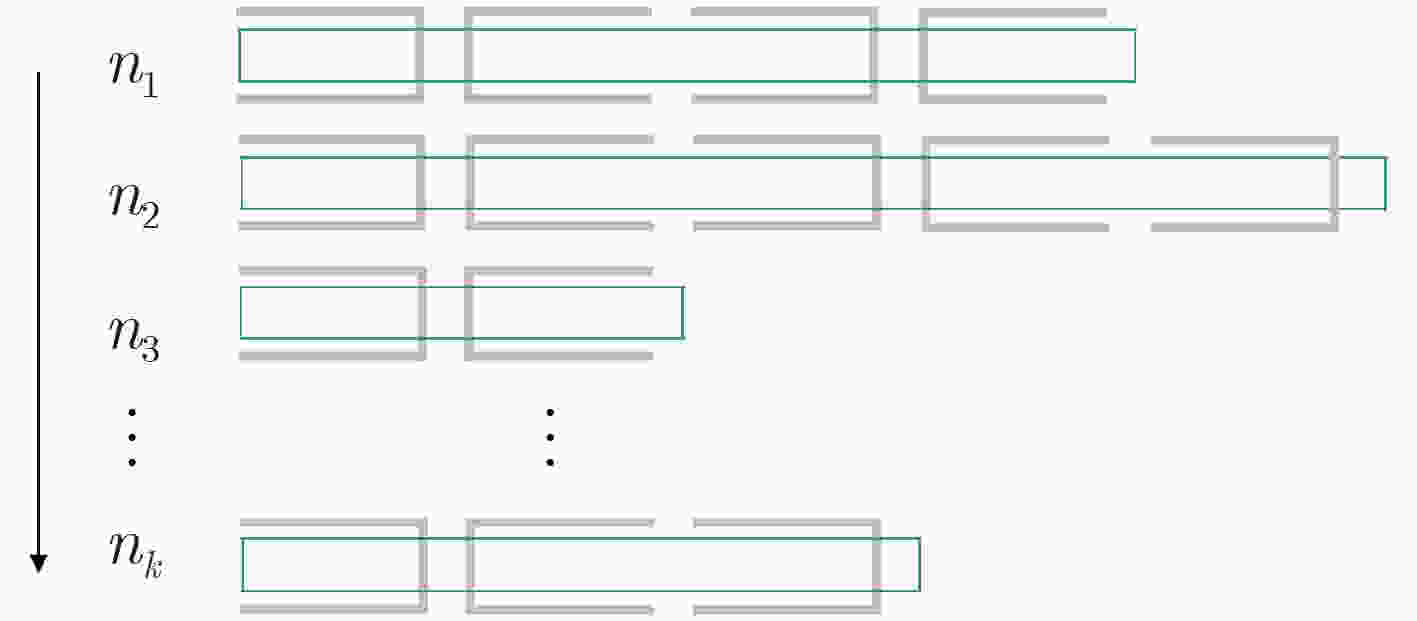


 下载:
下载:
