Methods for Measuring Single-Cell Structural and Electrical Properties
-
摘要: 单细胞固有生物物理学特征,主要包括结构特征如细胞直径和细胞核直径,以及电学特征如细胞膜比电容和细胞质电导率,已经被应用于细胞亚类型分类和细胞状态评估,在生物医学研究和临床诊断方面具有广阔的应用前景。该文综述了不同类型的单细胞结构和电学特征检测方法,介绍了固定式、流动式以及基于微流控的方法。归纳总结了这些方法的工作原理、发展和主要优缺点,探讨了单细胞结构和电学特征检测所面临的挑战以及未来的研究机遇。Abstract: Single-cell intrinsic biophysical properties including structural properties (e.g., cellular diameter and nuclear diameter) and electrical properties (e.g., specific membrane capacitance and cytoplasmic conductivity) have been applied to cell subtype classifications, and cell status evaluations are promising in biomedical research and clinical diagnosis. Different types of single-cell structural and electrical properties detection methods are reviewed in this paper, then fixed and flow methods and microfluidic methods are introduced. The working principles, developments, main advantages and disadvantages of these methods are summarized, and the challenges and future research opportunities for measurements of single-cell structural and electrical properties are discussed.
-
Key words:
- Single-cell analysis /
- Structural properties /
- Electrical properties /
- Microfluidics
-
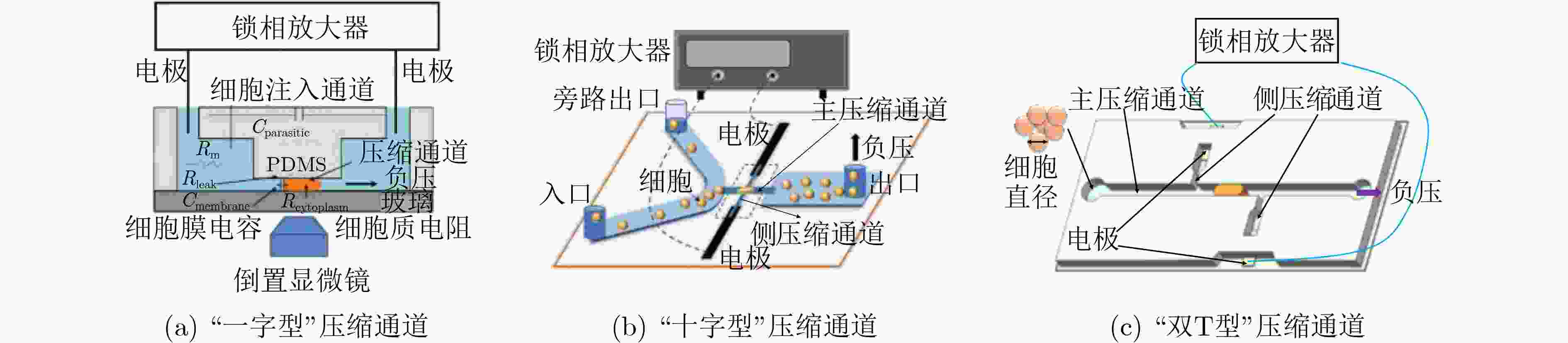
表 1 单细胞结构和电学特征检测方法标志性发展
方法 检测参数 区分目标细胞/关键成就 参考文献 固定式 膜片钳 细胞膜电容 表征数十个细胞电学特征 [30] 电旋转 细胞膜比电容 表征数百个细胞电学特征 [47] 介电电泳 细胞膜比电容和细胞质电导率 表征细胞群体平均电学特征 [55] 流动式 电阻抗 直流阻抗数据;直流+交流阻抗数据 白细胞3分群;白细胞5分类 [56] 光散射 前向+侧向散射光;多角度散射光 白细胞3分群;白细胞5分类 [56] 光成像 荧光成像 表征细胞直径和细胞核直径 [67] 微流控方法 共面电极 双频阻抗数据 5, 8 μm珠子;红细胞及其血影细胞 [68] 对面电极 双频阻抗数据 5, 6 μm珠子;红细胞及其固定细胞 [69] 对面电极+光学透镜 双频阻抗数据和荧光信号 白细胞3分群 [70] 多电极 单频阻抗数据;多频阻抗数据 5, 6, 7, 10 μm珠子;红细胞;酵母细胞 [72-77] “一字型”压缩通道 细胞膜比电容、质电导率和细胞直径 表征数百个肿瘤细胞固有电学特征 [78] “十字型”压缩通道 细胞膜比电容和细胞质电导率 表征数十万个肿瘤细胞固有电学特征 [79] “双T型”压缩通道 细胞膜比电容、质电导率和细胞直径 表征数十万个肿瘤细胞固有生物电学特征 [80] -
[1] HAN Xiaojun, BERKEL C, GWYER J, et al. Microfluidic lysis of human blood for leukocyte analysis using single cell impedance cytometry[J]. Analytical Chemistry, 2012, 84(2): 1070–1075. doi: 10.1021/ac202700x [2] DU E, HA S, DIEZ-SILVA M, et al. Electric impedance microflow cytometry for characterization of cell disease states[J]. Lab on A Chip, 2013, 13(19): 3903–3909. doi: 10.1039/c3lc50540e [3] NWANKIRE C E, VENKATANARAYANAN A, GLENNON T, et al. Label-free impedance detection of cancer cells from whole blood on an integrated centrifugal microfluidic platform[J]. Biosensors and Bioelectronics, 2015, 68: 382–389. doi: 10.1016/j.bios.2014.12.049 [4] TRAN A K, SAPKOTA A, WEN Jianming, et al. Linear relationship between cytoplasm resistance and hemoglobin in red blood cell hemolysis by electrical impedance spectroscopy & eight-parameter equivalent circuit[J]. Biosensors and Bioelectronics, 2018, 119: 103–109. doi: 10.1016/j.bios.2018.08.012 [5] RAILLON C, CHE J, THILL S, et al. Toward microfluidic label-free isolation and enumeration of circulating tumor cells from blood samples[J]. Cytometry Part A, 2019, 95(10): 1085–1095. doi: 10.1002/cyto.a.23868 [6] GRIFFITHS T M, PAGE L, WEYRICH A S, et al. Platelet electrical resistance for measuring platelet activation and adhesion in human health and disease[J]. Thrombosis Research, 2021, 198: 204–209. doi: 10.1016/j.thromres.2020.12.012 [7] LIU Jia, QIANG Yuhao, and DU E. Dielectric spectroscopy of red blood cells in sickle cell disease[J]. Electrophoresis, 2021, 42(5): 667–675. doi: 10.1002/elps.202000143 [8] MAN Yuncheng, MAJI D, AN Ran, et al. Microfluidic electrical impedance assessment of red blood cell-mediated microvascular occlusion[J]. Lab on A Chip, 2021, 21(6): 1036–1048. doi: 10.1039/d0lc01133a [9] COLEY H M, LABEED F H, THOMAS H, et al. Biophysical characterization of MDR breast cancer cell lines reveals the cytoplasm is critical in determining drug sensitivity[J]. Biochimica et Biophysica Acta (BBA)-General Subjects, 2007, 1770(4): 601–608. doi: 10.1016/j.bbagen.2006.12.002 [10] LIANG X, GRAHAM K A, JOHANNESSEN A C, et al. Human oral cancer cells with increasing tumorigenic abilities exhibit higher effective membrane capacitance[J]. Integrative Biology, 2014, 6(5): 545–554. doi: 10.1039/C3IB40255J [11] ZHAO Yang, ZHAO Xiaoting, CHEN Deyong, et al. Tumor cell characterization and classification based on cellular specific membrane capacitance and cytoplasm conductivity[J]. Biosensors and Bioelectronics, 2014, 57: 245–253. doi: 10.1016/j.bios.2014.02.026 [12] KUMAR R T K, LIU Shanshan, MINNA J D, et al. Monitoring drug induced apoptosis and treatment sensitivity in non-small cell lung carcinoma using dielectrophoresis[J]. Biochimica et Biophysica Acta (BBA)-General Subjects, 2016, 1860(9): 1877–1883. doi: 10.1016/j.bbagen.2016.05.039 [13] TANG Wenlai, TANG Dezhi, NI Zhonghua, et al. Microfluidic impedance cytometer with inertial focusing and liquid electrodes for high-throughput cell counting and discrimination[J]. Analytical Chemistry, 2017, 89(5): 3154–3161. doi: 10.1021/acs.analchem.6b04959 [14] SANO M, KAJI N, ROWAT A C, et al. Microfluidic mechanotyping of a single cell with two consecutive constrictions of different sizes and an electrical detection system[J]. Analytical Chemistry, 2019, 91(20): 12890–12899. doi: 10.1021/acs.analchem.9b02818 [15] DABIGHI A and TOGHRAIE D. A new microfluidic device for separating circulating tumor cells based on their physical properties by using electrophoresis and dielectrophoresis forces within an electrical field[J]. Computer Methods and Programs in Biomedicine, 2020, 185: 105147. doi: 10.1016/j.cmpb.2019.105147 [16] HOSSAIN S. Malignant cell characterization via mathematical analysis of bio impedance and optical properties[J]. Electromagnetic Biology and Medicine, 2021, 40(1): 65–83. doi: 10.1080/15368378.2020.1850471 [17] SONG Hongjun, WANG Yi, ROSANO J M, et al. A microfluidic impedance flow cytometer for identification of differentiation state of stem cells[J]. Lab on A Chip, 2013, 13(12): 2300–2310. doi: 10.1039/c3lc41321g [18] ZHOU Ying, BASU S, LAUE E, et al. Single cell studies of mouse embryonic stem cell (mESC) differentiation by electrical impedance measurements in a microfluidic device[J]. Biosensors and Bioelectronics, 2016, 81: 249–258. doi: 10.1016/j.bios.2016.02.069 [19] XAVIER M, DE ANDRÉS M C D, SPENCER D, et al. Size and dielectric properties of skeletal stem cells change critically after enrichment and expansion from human bone marrow: Consequences for microfluidic cell sorting[J]. Journal of the Royal Society Interface, 2017, 14(133): 20170233. doi: 10.1098/rsif.2017.0233 [20] EL-BATTRAWY I, ZHAO Zhilan, LAN Huan, et al. Estradiol protection against toxic effects of catecholamine on electrical properties in human-induced pluripotent stem cell derived cardiomyocytes[J]. International Journal of Cardiology, 2018, 254: 195–202. doi: 10.1016/j.ijcard.2017.11.007 [21] ZHOU Wenli, GRAHAM K, LUCENDO-VILLARIN B, et al. Combining stem cell-derived hepatocytes with impedance sensing to better predict human drug toxicity[J]. Expert Opinion on Drug Metabolism & Toxicology, 2019, 15(1): 77–83. doi: 10.1080/17425255.2019.1558208 [22] ZHANG Zhizhong, ZHENG Tianyang, and ZHU Rong. Microchip with single-cell impedance measurements for monitoring osteogenic differentiation of mesenchymal stem cells under electrical stimulation[J]. Analytical Chemistry, 2020, 92(18): 12579–12587. doi: 10.1021/acs.analchem.0c02556 [23] LEI Kinfong, HO Y C, HUANG C H, et al. Characterization of stem cell-like property in cancer cells based on single-cell impedance measurement in a microfluidic platform[J]. Talanta, 2021, 229: 122259. doi: 10.1016/J.TALANTA.2021.122259 [24] GRAVESEN P, BRANEBJERG J, and JENSEN O S. Microfluidics-a review[J]. Journal of Micromechanics and Microengineering, 1993, 3(4): 168–182. doi: 10.1088/0960-1317/3/4/002 [25] REECE A, XIA Bingzhao, JIANG Zhongliang, et al. Microfluidic techniques for high throughput single cell analysis[J]. Current Opinion in Biotechnology, 2016, 40: 90–96. doi: 10.1016/j.copbio.2016.02.015 [26] GOLOWASCH J, THOMAS G, TAYLOR A L, et al. Membrane capacitance measurements revisited: Dependence of capacitance value on measurement method in nonisopotential neurons[J]. Journal of Neurophysiology, 2009, 102(4): 2161–2175. doi: 10.1152/jn.00160.2009 [27] SAKABA T, HAZAMA A, and MARUYAMA Y. Patch-clamp capacitance measurements[M]. OKADA Y. Patch clamp techniques: From Beginning to Advanced Protocols. Tokyo, 2012: 277–286. [28] NEHER E and MARTY A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells[J]. Proceedings of the National Academy of Sciences of the United States of America, 1982, 79(21): 6712–6716. doi: 10.1073/pnas.79.21.6712 [29] FERNANDEZ J M, NEHER E, and GOMPERTS B D. Capacitance measurements reveal stepwise fusion events in degranulating mast cells[J]. Nature, 1984, 312(5993): 453–455. doi: 10.1038/312453a0 [30] LINDAU M and NEHER E. Patch-clamp techniques for time-resolved capacitance measurements in single cells[J]. Pflügers Archiv, 1988, 411(2): 137–146. doi: 10.1007/BF00582306 [31] DONNELLY D F. A novel method for rapid measurement of membrane resistance, capacitance, and access resistance[J]. Biophysical Journal, 1994, 66(3): 873–877. doi: 10.1016/S0006-3495(94)80863-X [32] ROHLICEK V and SCHMID A. Dual-frequency method for synchronous measurement of cell capacitance, membrane conductance and access resistance on single cells[J]. Pflügers Archiv, 1994, 428(1): 30–38. doi: 10.1007/BF00374749 [33] O'SHAUGHNESSY T J and KIM Y I. A computer-based system for the measurement of membrane capacitance to monitor exocytosis in secretory cells[J]. Journal of Neuroscience Methods, 1995, 57(1): 1–8. doi: 10.1016/0165-0270(94)00104-O [34] NEEF A, HEINEMANN C, and MOSER T. Measurements of membrane patch capacitance using a software-based lock-in system[J]. Pflügers Archiv, 2007, 454(2): 335–344. doi: 10.1007/s00424-006-0191-1 [35] CHEN Peng and GILLIS K D. The noise of membrane capacitance measurements in the whole-cell recording configuration[J]. Biophysical Journal, 2000, 79(4): 2162–2170. doi: 10.1016/S0006-3495(00)76464-2 [36] ZHANG Hao, QU Anlian, LUO Jie, et al. Error analysis of Cm measurement under the whole-cell patch-clamp recording[J]. Journal of Neuroscience Methods, 2010, 185(2): 307–314. doi: 10.1016/j.jneumeth.2009.10.003 [37] KODANDARAMAIAH S B, FRANZESI G T, CHOW B Y, et al. Automated whole-cell patch-clamp electrophysiology of neurons in vivo[J]. Nature Methods, 2012, 9(6): 585–587. doi: 10.1038/nmeth.1993 [38] FRANZ D, OLSEN H L, KLINK O, et al. Automated and manual patch clamp data of human induced pluripotent stem cell-derived dopaminergic neurons[J]. Scientific Data, 2017, 4(1): 170056. doi: 10.1038/sdata.2017.56 [39] GOATER A D and PETHIG R. Electrorotation and dielectrophoresis[J]. Parasitology, 1998, 117 Suppl: S177–S189. [40] GIMSA J. A comprehensive approach to electro-orientation, electrodeformation, dielectrophoresis, and electrorotation of ellipsoidal particles and biological cells[J]. Bioelectrochemistry, 2001, 54(1): 23–31. doi: 10.1016/S0302-4598(01)00106-4 [41] ARNOLD W M and ZIMMERMANN U. Rotating-field-induced rotation and measurement of the membrane capacitance of single mesophyll cells of Avena sativa[J]. Zeitschrift Für Naturforschung C, 1982, 37(10): 908–915. doi: 10.1515/znc-1982-1010 [42] ARNOLD W M, WENDT B, ZIMMERMANN U, et al. Rotation of a single swollen thylakoid vesicle in a rotating electric field. Electrical properties of the photosynthetic membrane and their modification by ionophores, lipophilic ions and pH[J]. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1985, 813(1): 117–131. doi: 10.1016/0005-2736(85)90352-9 [43] FUHR G, GLASER R, and HAGEDORN R. Rotation of dielectrics in a rotating electric high-frequency field. Model experiments and theoretical explanation of the rotation effect of living cells[J]. Biophysical Journal, 1986, 49(2): 395–402. doi: 10.1016/S0006-3495(86)83649-9 [44] FUHR G and KUZMIN P I. Behavior of cells in rotating electric fields with account to surface charges and cell structures[J]. Biophysical Journal, 1986, 50(5): 789–795. doi: 10.1016/S0006-3495(86)83519-6 [45] HUGHES M P, WANG X B, BECKER F F, et al. Computer-aided analyses of electric fields used in electrorotation studies[J]. Journal of Physics D:Applied Physics, 1994, 27(7): 1564–1570. doi: 10.1088/0022-3727/27/7/035 [46] HUGHES M P. Computer-aided analysis of conditions for optimizing practical electrorotation[J]. Physics in Medicine and Biology, 1998, 43(12): 3639–3648. doi: 10.1088/0031-9155/43/12/019 [47] DE GASPERIS G, WANG Xiaobo, YANG Jun, et al. Automated electrorotation: Dielectric characterization of living cells by real-time motion estimation[J]. Measurement Science and Technology, 1998, 9(3): 518–529. doi: 10.1088/0957-0233/9/3/029 [48] ZHOU X F, BURT J P H, and PETHIG R. Automatic cell electrorotation measurements: Studies of the biological effects of low-frequency magnetic fields and of heat shock[J]. Physics in Medicine and Biology, 1998, 43(5): 1075–1090. doi: 10.1088/0031-9155/43/5/003 [49] CRISTOFANILLI M, DE GASPERIS G, ZHANG Lisha, et al. Automated electrorotation to reveal dielectric variations related to HER-2/neu overexpression in MCF-7 sublines[J]. Clinical Cancer Research, 2002, 8(2): 615–619. [50] MIETCHEN D, SCHNELLE T, MÜLLER T, et al. Automated dielectric single cell spectroscopy- temperature dependence of electrorotation[J]. Journal of Physics D:Applied Physics, 2002, 35(11): 1258–1270. doi: 10.1088/0022-3727/35/11/324 [51] BECKER F F, WANG Xujing, HUANG Y, et al. Separation of human breast cancer cells from blood by differential dielectric affinity[J]. Proceedings of the National Academy of Sciences of the United States of America, 1995, 92(3): 860–864. doi: 10.1073/pnas.92.3.860 [52] LANNIN T, SU W W, GRUBER C, et al. Automated electrorotation shows electrokinetic separation of pancreatic cancer cells is robust to acquired chemotherapy resistance, serum starvation, and EMT[J]. Biomicrofluidics, 2016, 10(6): 064109. doi: 10.1063/1.4964929 [53] HU Xun, ARNOLD W M, and ZIMMERMANN U. Alterations in the electrical properties of T and B lymphocyte membranes induced by mitogenic stimulation. Activation monitored by electro-rotation of single cells[J]. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1990, 1021(2): 191–200. doi: 10.1016/0005-2736(90)90033-K [54] YANG Jun, HUANG Ying, WANG Xujing, et al. Dielectric properties of human leukocyte subpopulations determined by electrorotation as a cell separation criterion[J]. Biophysical Journal, 1999, 76(6): 3307–3314. doi: 10.1016/S0006-3495(99)77483-7 [55] LABEED F H, COLEY H M, THOMAS H, et al. Assessment of multidrug resistance reversal using dielectrophoresis and flow cytometry[J]. Biophysical Journal, 2003, 85(3): 2028–2034. doi: 10.1016/S0006-3495(03)74630-X [56] DENICOLA P D B. Advances in hematology analyzers[J]. Topics in Companion Animal Medicine, 2011, 26(2): 52–61. doi: 10.1053/j.tcam.2011.02.001 [57] CHOI H, KIM K B, JEON C S, et al. A label-free DC impedance-based microcytometer for circulating rare cancer cell counting[J]. Lab on A Chip, 2013, 13(5): 970–977. doi: 10.1039/c2lc41376k [58] RHO J, JANG W, HWANG I, et al. Multiplex immunoassays using virus-tethered gold microspheres by DC impedance-based flow cytometry[J]. Biosensors and Bioelectronics, 2017, 102: 121–128. doi: 10.1016/j.bios.2017.11.027 [59] SIMON P, FRANKOWSKI M, BOCK N, et al. Label-free whole blood cell differentiation based on multiple frequency AC impedance and light scattering analysis in a micro flow cytometer[J]. Lab on A Chip, 2016, 16(12): 2326–2338. doi: 10.1039/c6lc00128a [60] CAREY T R, COTNER K L, LI B, et al. Developments in label-free microfluidic methods for single-cell analysis and sorting[J]. WIREs:Nanomedicine and Nanobiotechnology, 2019, 11(1): e1529. doi: 10.1002/wnan.1529 [61] TERSTAPPEN L W M M, DE GROOTH B G, TEN NAPEL C H H, et al. Discrimination of human cytotoxic lymphocytes from regulatory and B-lymphocytes by orthogonal light scattering[J]. Journal of Immunological Methods, 1986, 95(2): 211–216. doi: 10.1016/0022-1759(86)90408-4 [62] CIFANI N, PROIETTA M, TAURINO M, et al. Monocyte Subsets, stanford-a acute aortic dissection, and carotid artery stenosis: New evidences[J]. Journal of Immunology Research, 2019, 2019: 9782594. doi: 10.1155/2019/9782594 [63] DANNHAUSER D, ROSSI D, RIPALDI M, et al. Single-cell screening of multiple biophysical properties in leukemia diagnosis from peripheral blood by pure light scattering[J]. Scientific Reports, 2017, 7(1): 12666. doi: 10.1038/s41598-017-12990-4 [64] SCHMIT T, KLOMP M, and KHAN M N. An overview of flow cytometry: Its principles and applications in allergic disease research[M]. NAGAMOTO-COMBS K. Animal Models of Allergic Disease: Methods and Protocols. New York, USA, 2021, 2223: 169–182. [65] RUBAN G I, KOSMACHEVA S M, GONCHAROVA N V, et al. Investigation of morphometric parameters for granulocytes and lymphocytes as applied to a solution of direct and inverse light-scattering problems[J]. Journal of Biomedical Optics, 2007, 12(4): 044017. doi: 10.1117/1.2753466 [66] LIU Shanshan, YUAN Zeng, QIAO Xu, et al. Light scattering pattern specific convolutional network static cytometry for label-free classification of cervical cells[J]. Cytometry Part A, 2021, 99(6): 610–621. doi: 10.1002/cyto.a.24349 [67] STAVRAKIS S, HOLZNER G, CHOO J, et al. High-throughput microfluidic imaging flow cytometry[J]. Current Opinion in Biotechnology, 2019, 55: 36–43. doi: 10.1016/j.copbio.2018.08.002 [68] GAWAD S, SCHILD L, and RENAUD P. Micromachined impedance spectroscopy flow cytometer for cell analysis and particle sizing[J]. Lab on A Chip, 2001, 1(1): 76–82. doi: 10.1039/b103933b [69] CHEUNG K, GAWAD S, and RENAUD P. Impedance spectroscopy flow cytometry: On-chip label-free cell differentiation[J]. Cytometry Part A, 2005, 65A(2): 124–132. doi: 10.1002/cyto.a.20141 [70] HOLMES D, PETTIGREW D, RECCIUS C H, et al. Leukocyte analysis and differentiation using high speed microfluidic single cell impedance cytometry[J]. Lab on A Chip, 2009, 9(20): 2881–2889. doi: 10.1039/b910053a [71] HOLMES D and MORGAN H. Single cell impedance cytometry for identification and counting of CD4 T-cells in human blood using impedance labels[J]. Analytical Chemistry, 2010, 82(4): 1455–1461. doi: 10.1021/ac902568p [72] CASELLI F and BISEGNA P. Simulation and performance analysis of a novel high-accuracy sheathless microfluidic impedance cytometer with coplanar electrode layout[J]. Medical Engineering & Physics, 2017, 48: 81–89. doi: 10.1016/j.medengphy.2017.04.005 [73] REALE R, DE NINNO A, BUSINARO L, et al. High-throughput electrical position detection of single flowing particles/cells with non-spherical shape[J]. Lab on A Chip, 2019, 19(10): 1818–1827. doi: 10.1039/C9LC00071B [74] HONRADO C, MCGRATH J S, REALE R, et al. A neural network approach for real-time particle/cell characterization in microfluidic impedance cytometry[J]. Analytical and Bioanalytical Chemistry, 2020, 412(16): 3835–3845. doi: 10.1007/s00216-020-02497-9 [75] YANG Dahou and AI Ye. Microfluidic impedance cytometry device with N-shaped electrodes for lateral position measurement of single cells/particles[J]. Lab on A Chip, 2019, 19(21): 3609–3617. doi: 10.1039/c9lc00819e [76] SPENCER D and MORGAN H. High-speed single-cell dielectric spectroscopy[J]. ACS Sensors, 2020, 5(2): 423–430. doi: 10.1021/acssensors.9b02119 [77] TANG Tao, LIU Xun, KIYA R, et al. Microscopic impedance cytometry for quantifying single cell shape[J]. Biosensors and Bioelectronics, 2021, 193: 113521. doi: 10.1016/j.bios.2021.113521 [78] ZHAO Yang, CHEN Deyong, LI Hao, et al. A microfluidic system enabling continuous characterization of single-cell specific membrane capacitance and cytoplasm conductivity[C]. The 8th Annual IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Suzhou, China, 2013: 344–347. [79] ZHAO Yang, WANG Ke, CHEN Deyong, et al. Development of microfluidic impedance cytometry enabling the quantification of specific membrane capacitance and cytoplasm conductivity from 100, 000 single cells[J]. Biosensors and Bioelectronics, 2018, 111: 138–143. doi: 10.1016/j.bios.2018.04.015 [80] ZHANG Yi, LIANG Hongyan, TAN Huiwen, et al. Development of microfluidic platform to high-throughput quantify single-cell intrinsic bioelectrical markers of tumor cell lines, subtypes and patient tumor cells[J]. Sensors and Actuators B:Chemical, 2020, 317: 128231. doi: 10.1016/j.snb.2020.128231 -





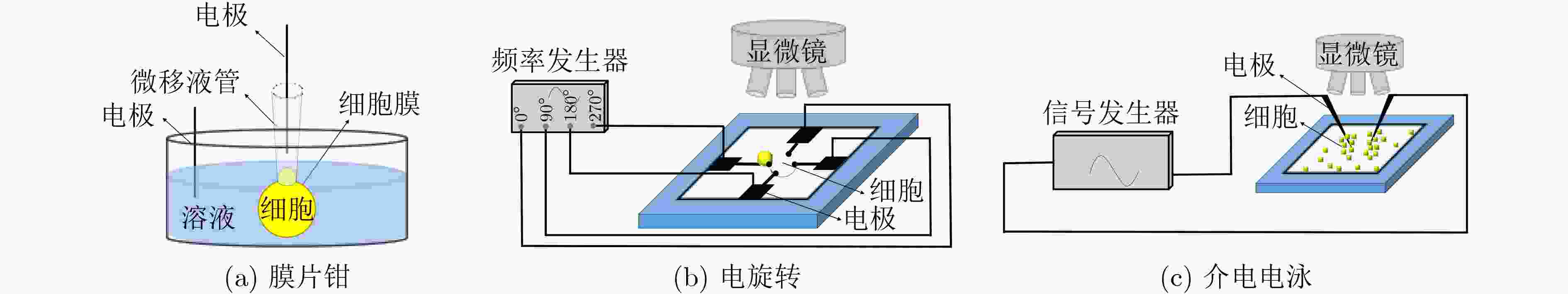
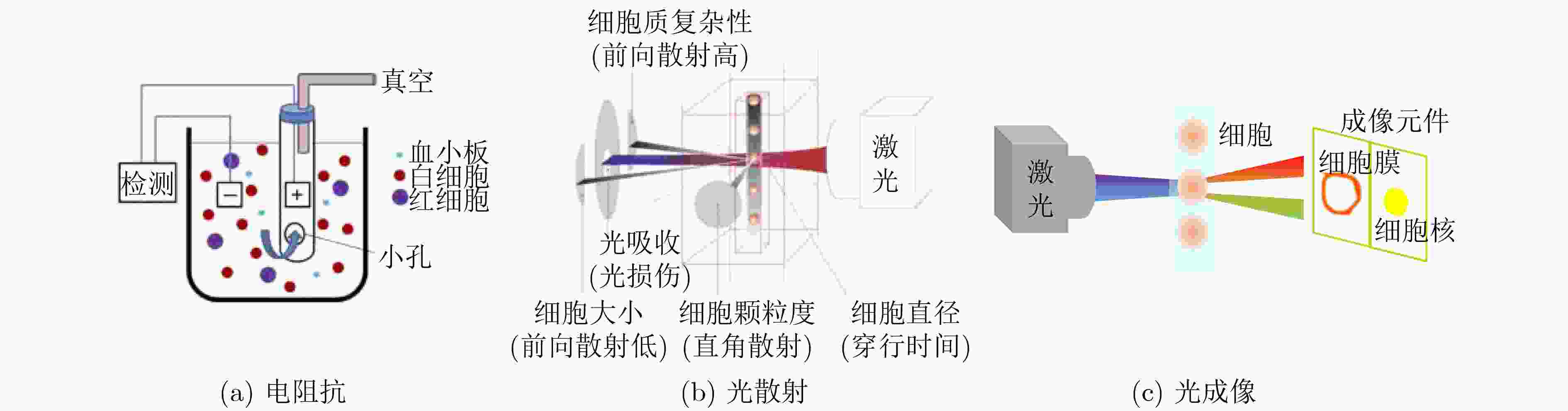
 下载:
下载:
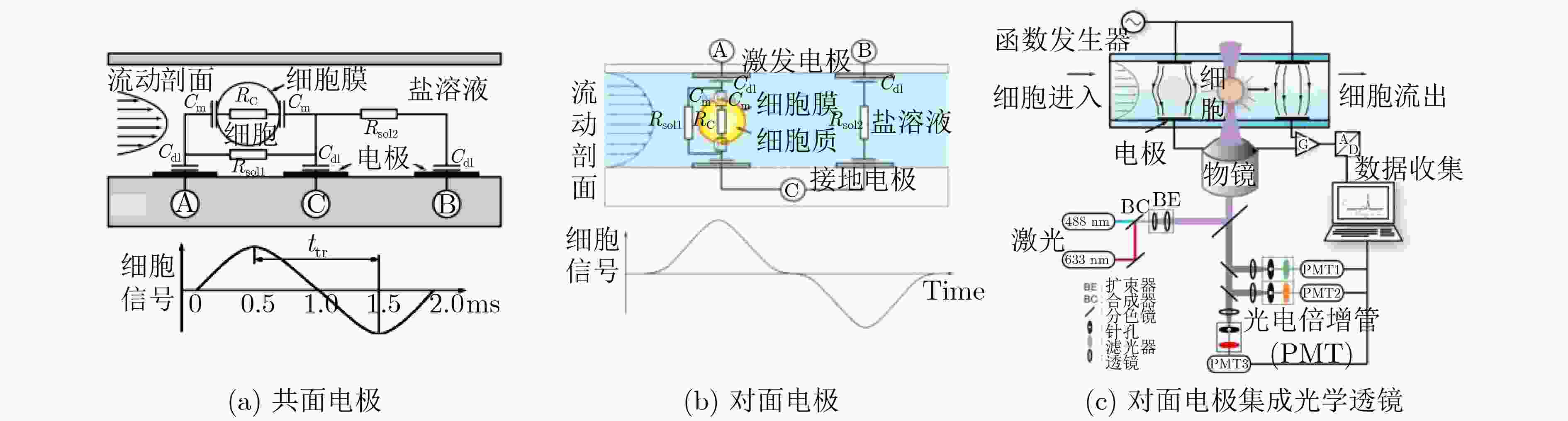
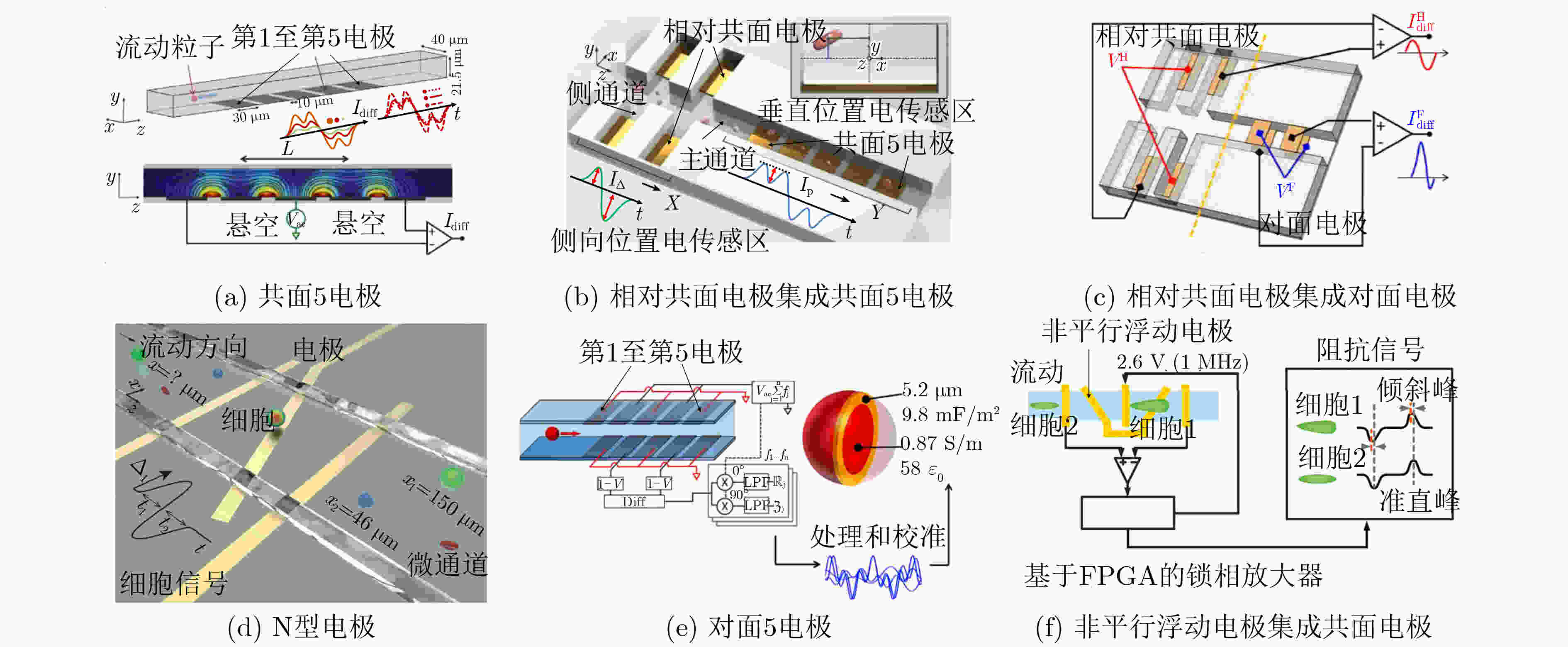


 下载:
下载:
